Creating a peripheral neural interface (PNI) that provides a stable integration of synthetic elements and neural tissue, is key to the development of true neuro-prostheses. The inevitable onset of reactive fibrosis around a synthetic implant is one of the major hurdles for long-term functionality of PNIs.
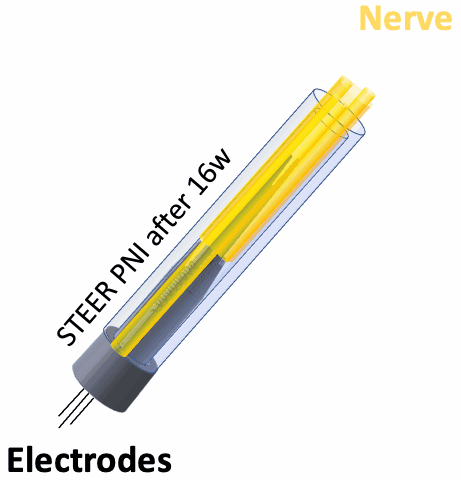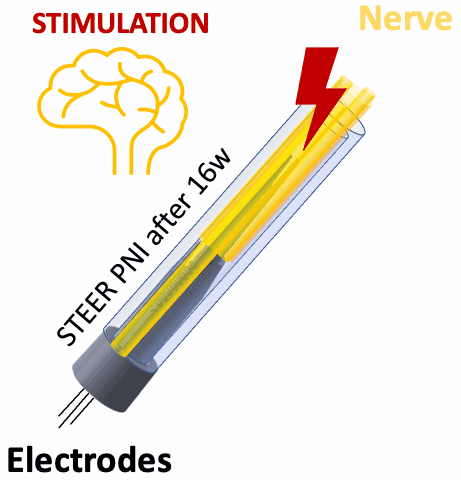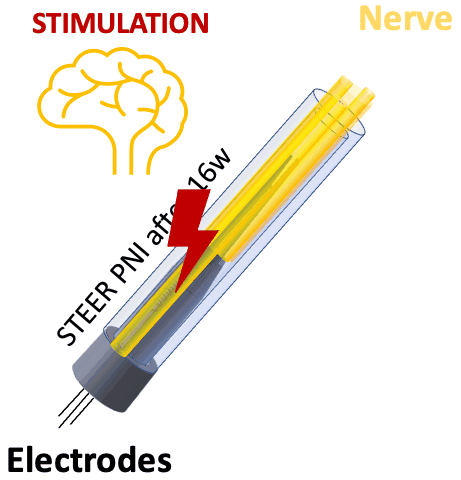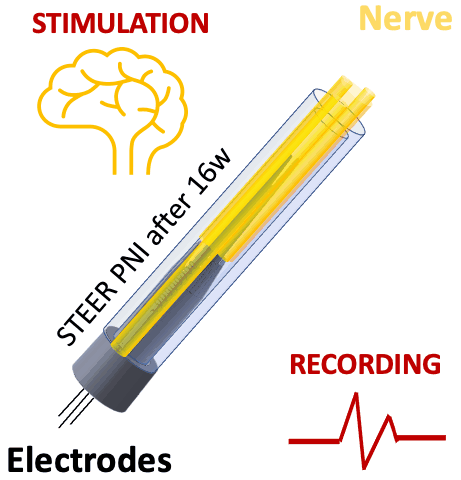
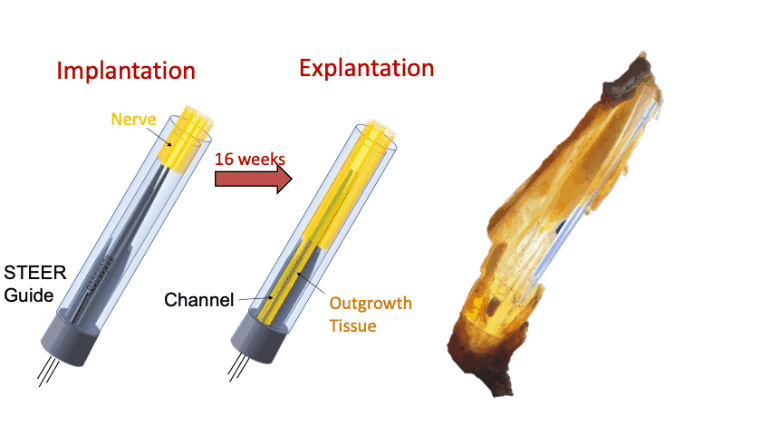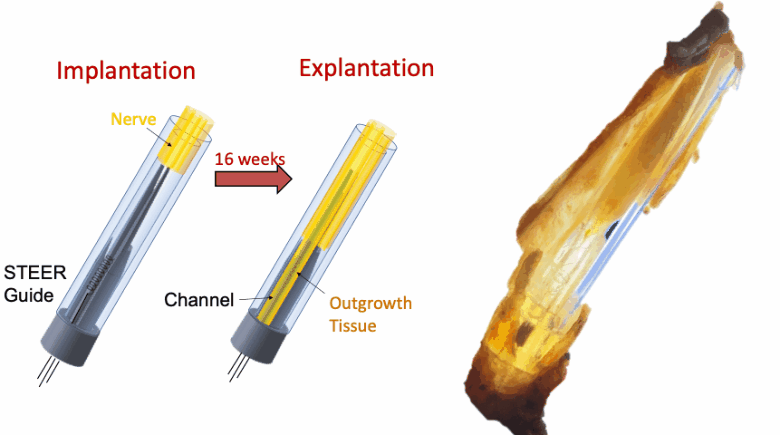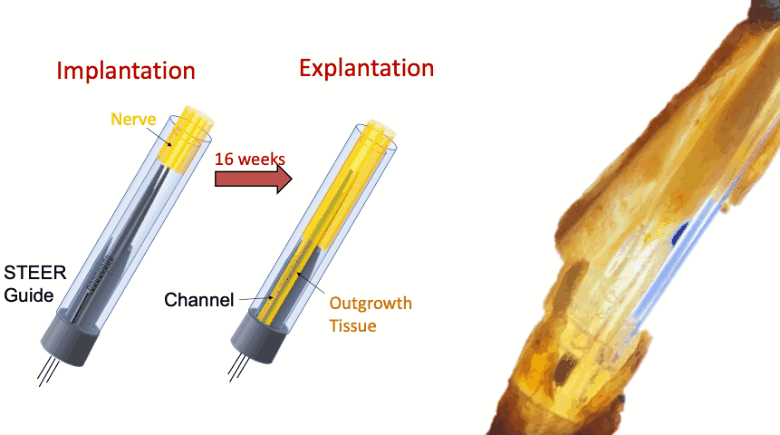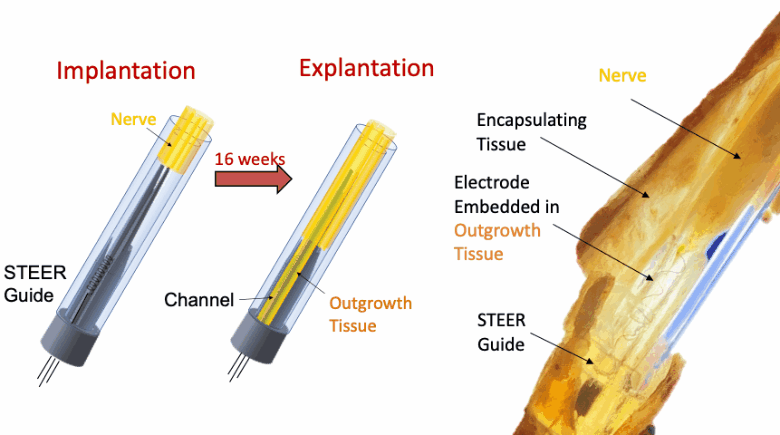
In this proof-of-concept study we created a PNI that harnessed the fibro-axonal growth, and incorporated fibrosis into the interface. The implant included a 3-D printed guide with a central spire and columns, forming channels that housed spring-like electrodes. The channels guided the fibro-axonal growth from the nerve towards encapsulation of the electrodes. We use the term STEER (Substrate-guided, Tissue-Electrode Encapsulation and Integration) PNI to represent this novel interface.
We tested STEER PNI in two non-human primates. Four months from implantation, we performed functional electrophysiological assessment and examined the macro- and micro-morphology of the interface. We successfully recorded a conduction of intracranially generated action potentials through STEER PNI. Morphology examinations revealed a distinct encapsulation of the STEER guide by the nerve’s fibro-axonal outgrowth, with uniquely configured laminae of myelinated axons incorporating the electrodes within the new tissue. The very low number of detected neutrophils suggest a lack of active inflammatory response and stability of the created construct between synthetic elements and neural tissue.
STEER implant reconfigured the structure of the fibro-axonal tissue and facilitated the formation of a stable interface between the tissue and the electrodes. The results point to the feasibility of our concept of STEER for creating a PNI with long-term electrophysiologic functionality by using a simple design and materials, which will find applications in neuro-prosthetics and beyond.