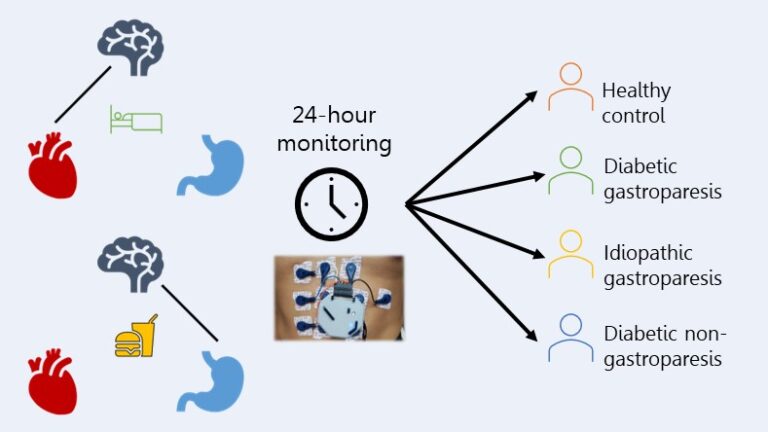
In this study, we collected multiple streams of physiologic data spanning both the autonomic nervous and digestive systems from healthy controls and patients with functional gastrointestinal disorders while they were at home for 24 hours. The autonomic nervous system is responsible for all unconscious functions, including heart rate, breathing, digestion, and sweating. The functional gastrointestinal disorder we focused on was gastroparesis, which is delayed gastric emptying without physical obstruction. Using these 24-hour data, we sought to identify differentiators in baseline autonomic and digestive function between healthy controls and patients and between different subtypes of patients.
To do so, we used a variety of different analyses techniques, each focused on extracting a specific type of information. One focused on heartbeat dynamics, computed from the electrocardiogram (ECG), to characterize autonomic activity to the heart throughout the day and night, representative of central changes in the autonomic nervous system. We also analyzed the frequency-related activity of the gastric slow wave, computed from the electrogastrogram (EGG), after meals, representative of the muscle-stimulating activity in the stomach itself.
We identified several key differentiators, specifically in regulation of autonomic activity throughout the circadian rhythm and in digestive activity patterns after meals, which separated patients from controls with an accuracy of 89% and separated specific patient phenotypes. For example, autonomic activity during sleep differentiated two different etiologies of gastroparesis, diabetic and idiopathic, from each other and healthy controls with an accuracy of 79%. In contrast, post-prandial (after meals) patterns in gastric slow wave power further differentiated diabetics with and without gastroparesis from each other with an accuracy of 90%. This work represents a first stage to identifying non-invasive biomarkers collected at home that could be used to verify functional gastrointestinal diagnoses, track disease progression for individuals, and suggest common disease origins between patients.