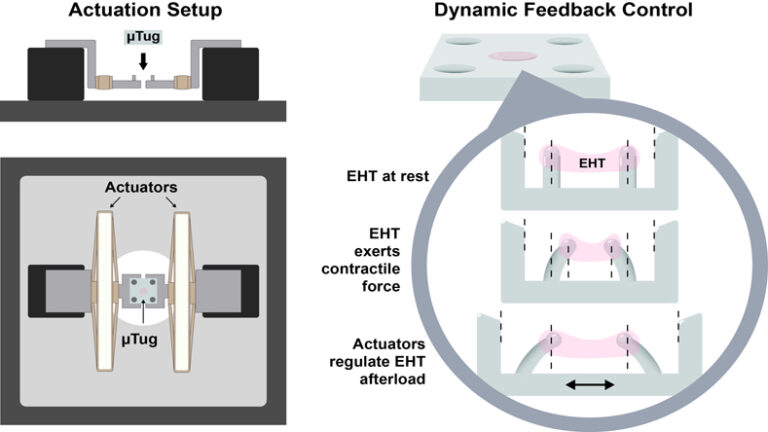
Three-dimensional engineered heart tissues (EHTs) derived from human induced pluripotent stem cells (iPSCs) have gained significant importance in drug toxicity screening and heart disease modeling. One crucial aspect of EHT characterization is the measurement of its contractile force, which indicates its ability to beat spontaneously. The contractility of cardiac muscle, its capacity to perform mechanical work, is governed by two key parameters: prestrain (preload) and external resistance (afterload). The effect of preload can be concisely generalized by the Frank-Starling law, while afterload remains less studied.
This study aims to demonstrate a technique for controlling afterload while monitoring the contractile force exerted by EHTs.
We have developed an apparatus capable of regulating the boundary conditions of EHTs in real-time through feedback control. The system comprises a pair of piezoelectric actuators that can strain the scaffold and a microscope that can measure the length of the EHTs and calculate the contractile force through image processing. By implementing closed-loop control, we can dynamically adjust the afterload of the EHTs.
When the boundary conditions are instantly switched from auxotonic to isometric, the twitch force of the EHTs immediately doubles. The changes in twitch force are characterized as a function of effective boundary stiffness. By comparing them to twitch force under auxotonic conditions, we found that the relative twitch force increases toward an asymptotic limit at higher stiffness ratios.
The capability to modify the mechanical boundary conditions of EHTs in real-time opens new avenues for investigating tissue mechanics. Our system serves as a suitable in vitro platform for mimicking pathologically induced afterload changes triggered by cardiac diseases or EHTs maturation with mechanical stimulations.