James D. Weiland and Mark S. Humayun, University of Southern California, Volume 61, Issue 5, page: 2629-2641
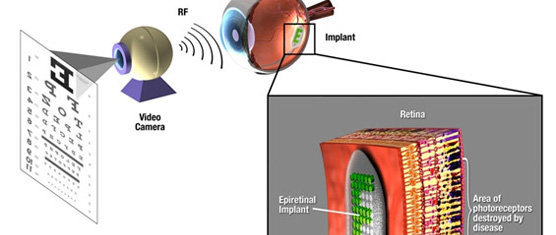
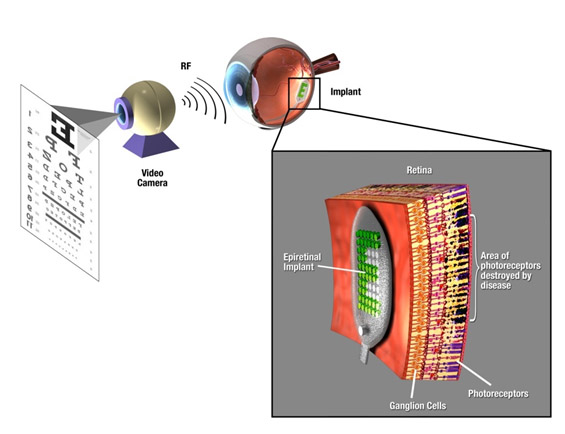 Retinal prostheses have been translated from the laboratory to the clinic over the past two decades. Currently, two devices have regulatory approval for the treatment of retinitis pigmentosa and several other devices have been tested in clinical trials. These devices provide partial sight restoration and patients use this improved vision in their everyday lives. Enhanced mobility and object detection are some of the more notable findings from the clinical trials. Patients with implants have demonstrated the ability to recognize letters as well, but the speed at which this is done does not correspond with reading using normal vision. To restore high resolution vision, both better technology and improved understanding of the interaction between electrical stimulation and the retina are needed. This review summarizes the recent clinical trials and highlights technology breakthroughs that will contribute to next generation of retinal prostheses. Significant advances have been made in integrated circuit design to allow systems with over 1000 individual outputs. Providing device power remains a challenge, but inductive link designs currently used are adequate, although more efficiency will enhance performance by reducing system size. A key technology that is limiting current implant is hermetic packaging. Current technology that can protect electronics for a decade is relatively large. Systems that rely on polymer coatings have lifetimes of less than 2 years. Thus, more research into materials and thin-film deposition is needed before bioelectronic retinal prostheses can reach their full potential.
Retinal prostheses have been translated from the laboratory to the clinic over the past two decades. Currently, two devices have regulatory approval for the treatment of retinitis pigmentosa and several other devices have been tested in clinical trials. These devices provide partial sight restoration and patients use this improved vision in their everyday lives. Enhanced mobility and object detection are some of the more notable findings from the clinical trials. Patients with implants have demonstrated the ability to recognize letters as well, but the speed at which this is done does not correspond with reading using normal vision. To restore high resolution vision, both better technology and improved understanding of the interaction between electrical stimulation and the retina are needed. This review summarizes the recent clinical trials and highlights technology breakthroughs that will contribute to next generation of retinal prostheses. Significant advances have been made in integrated circuit design to allow systems with over 1000 individual outputs. Providing device power remains a challenge, but inductive link designs currently used are adequate, although more efficiency will enhance performance by reducing system size. A key technology that is limiting current implant is hermetic packaging. Current technology that can protect electronics for a decade is relatively large. Systems that rely on polymer coatings have lifetimes of less than 2 years. Thus, more research into materials and thin-film deposition is needed before bioelectronic retinal prostheses can reach their full potential.