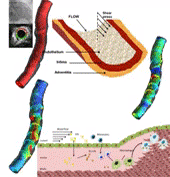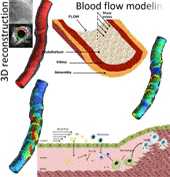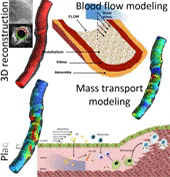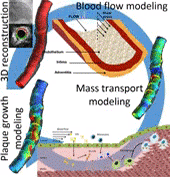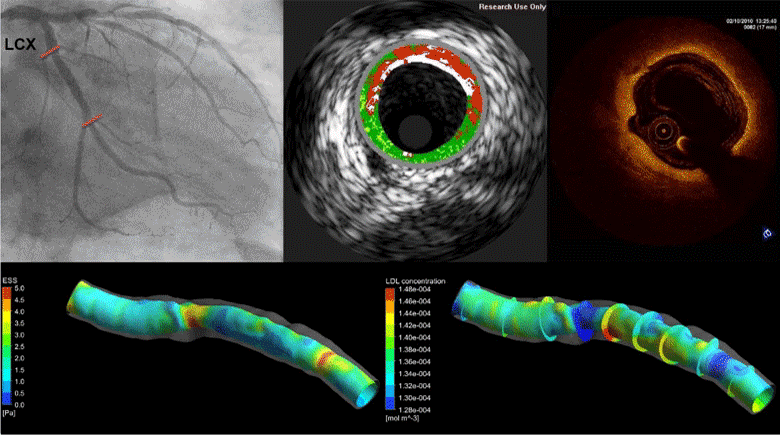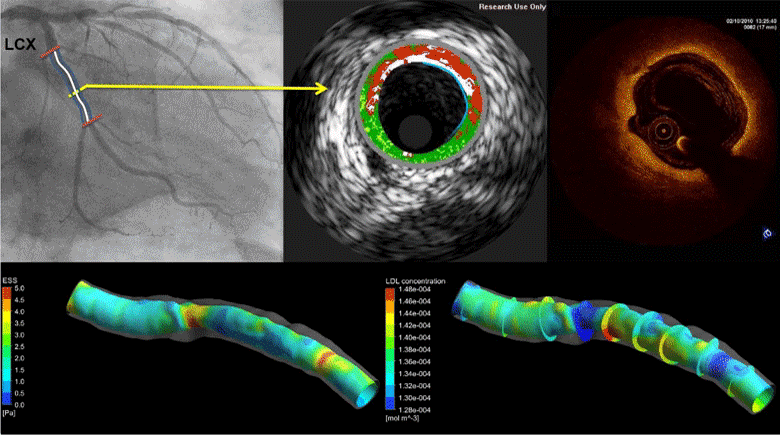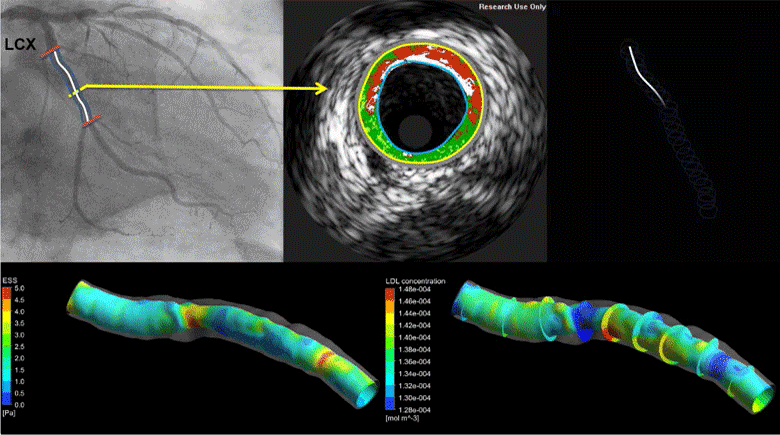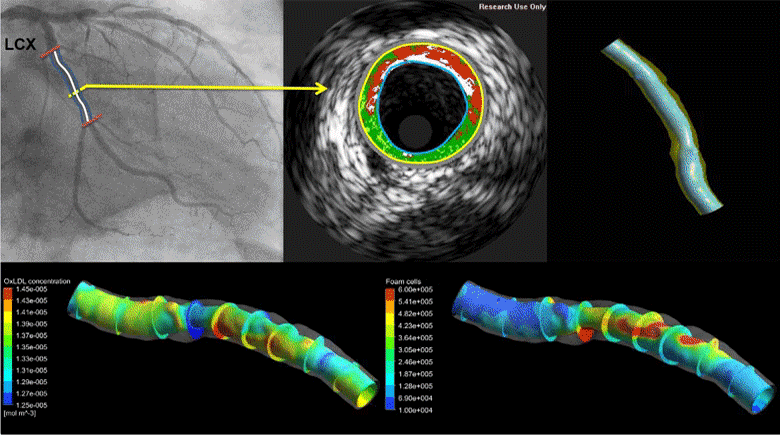
Coronary heart disease is considered one of the most common causes of death in west societies. The recent years low endothelial shear stress calculated using computational fluid dynamics has been found to be related with atherosclerotic plaque progression. However, until now it cannot be used for the prediction of regions, which are prone to growth. In this study, we implement a novel computational model, which simulates the major mechanisms of atherosclerotic plaque growth, using a proof-of-concept human arterial model. To this aim, a human reconstructed left circumflex coronary artery is utilized for a multi-level modeling approach. More specifically, the first level consists of the modeling of blood flow and endothelial shear stress (ESS) computation. The second level includes the modeling of low and high density lipoprotein (LDL, HDL) and monocytes transport through the endothelial membrane to vessel wall. The third level comprises of the modeling of LDL oxidation, macrophages differentiation and foam cells formation. The proposed approach is validated comparing the baseline computational results with the changes in arterial wall thickness, lumen diameter and plaque components as these are assessed at the follow-up examination. The results of this model show that ESS and LDL concentration have a good correlation with the changes in plaque area [R2=0.365 (P=0.029, adjusted R2=0.307) and R2=0.368 (P=0.015, adjusted R2=0.342), respectively] whereas the introduction of the variables of oxidized LDL, macrophages and foam cells as independent predictors improves the accuracy in predicting regions potential for atherosclerotic plaque development [R2=0.847 (P=0.009, adjusted R2=0.738)]. Concluding, computational models of plaque growth can be used to increase the accuracy to predict regions, which are prone to plaque development.