Adherent cell behaviors are shaped by a mix of intrinsic and extrinsic elements, including chemical and mechanical influences. These signals drive key cellular activities and are especially influential in tissues like lungs, arteries, cartilage, and the musculoskeletal system. Mechanical stimuli parameters significantly influence the biological response. Emulating these complex mechanical stimuli is vital to understand mechanobiology in healthy and disease states, such as cardiac fibrosis, idiopathic pulmonary fibrosis, musculoskeletal disorders, and connective tissue diseases.
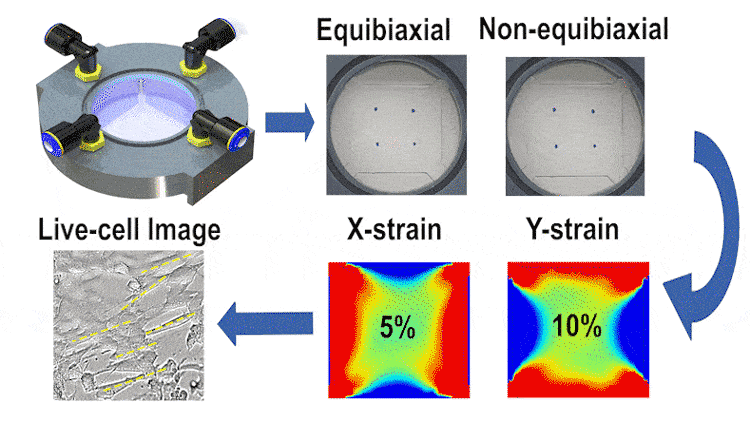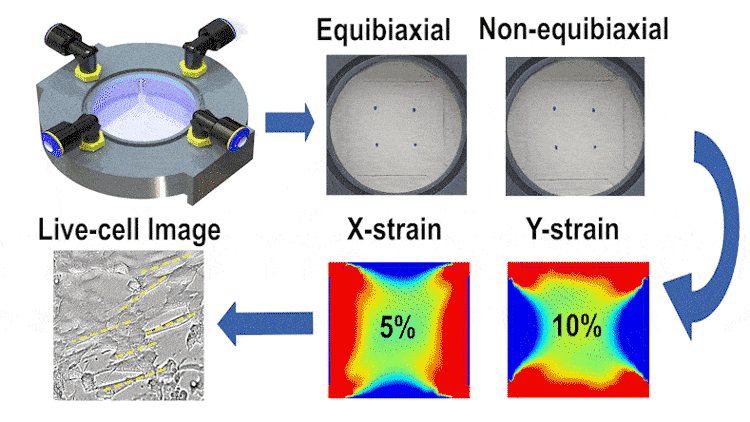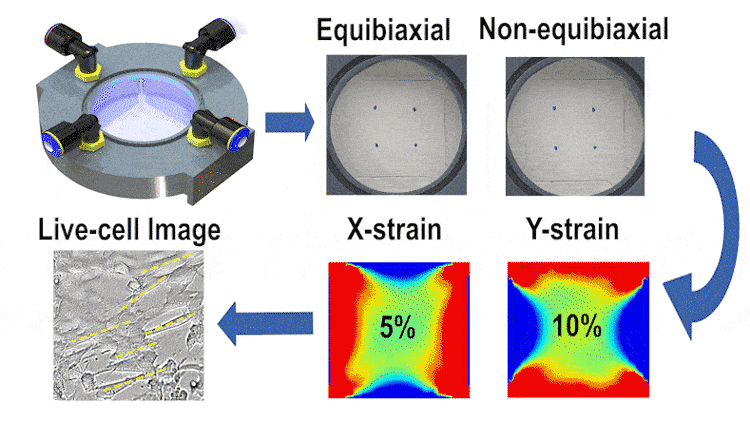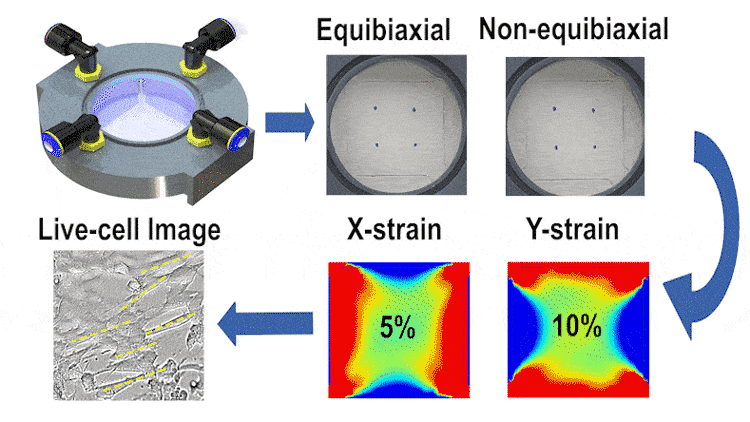
In this study, we engineered a cost-effective, pneumatically-controlled cell stretcher capable of delivering independent strain in two orthogonal directions of a membrane, thus offering a unique approach to non-equibiaxial stretching. This feature is critical in simulating the multifaceted mechanical environment cells encounter in vivo. The device, designed to fit within the scale of a 60-mm petri dish, enables large-area uniform stretching and is complemented by high-resolution optical imaging capabilities for real-time data observation.
We employed finite element simulations alongside image processing techniques for validating the strain fields and examining cell orientation and morphology under different stretching conditions. Our results indicate that the device can reliably apply up to 15% strain in each direction at 1 Hz, with a minimal strain measurement error. The differential stretching patterns induced notable variations in cell behavior, particularly in terms of cell length and area changes.
This study is the first of its kind to integrate such a high degree of control in cell biomechanics studies with real-time imaging, offering a significant leap forward in our ability to emulate and study complex mechanical stimuli on cells. The implications of this research are vast, potentially aiding in the deeper understanding of mechanobiology and fostering advancements in related biomedical fields.