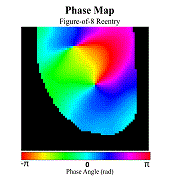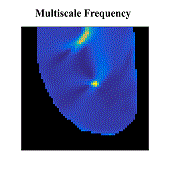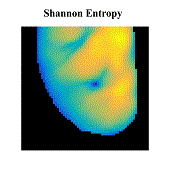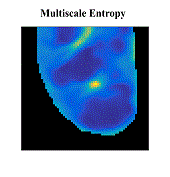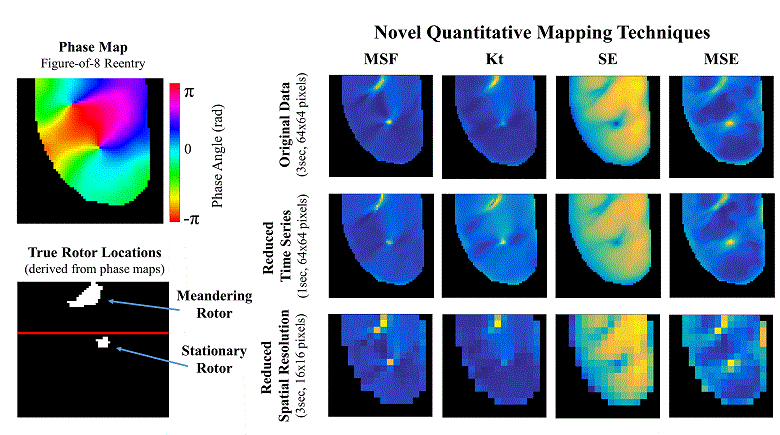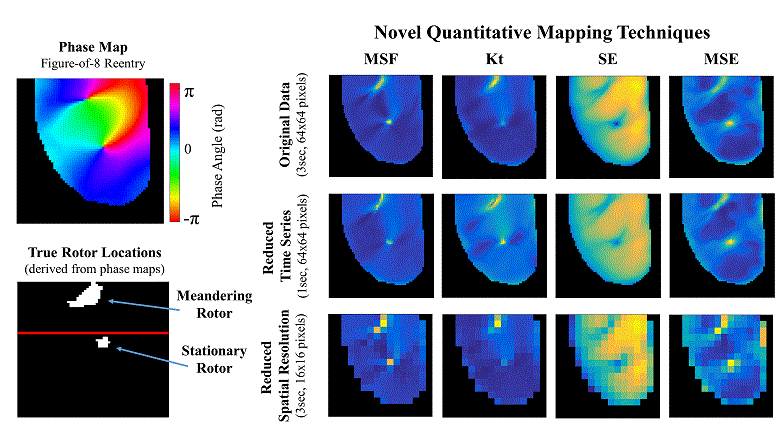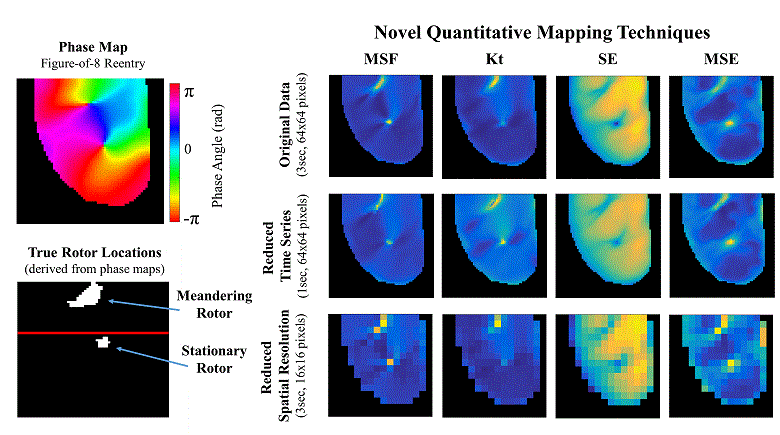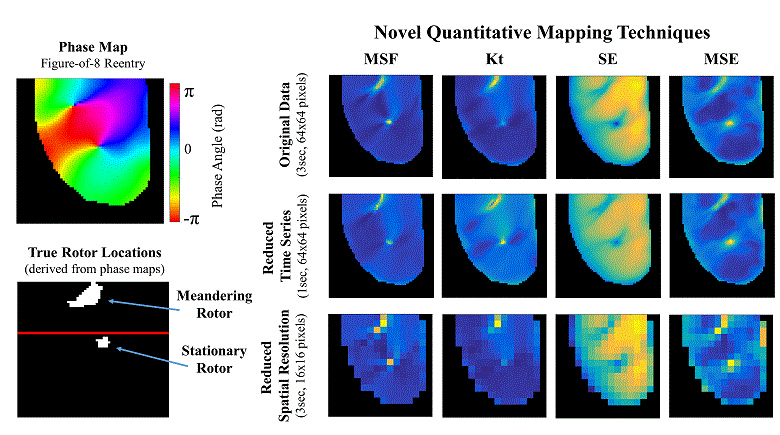
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in humans, which is believed to be maintained by rapid reentry sources (rotors). One treatment option is catheter ablation, which targets the tissue responsible for the AF initiation and maintenance, i.e. pivot points of rotors. Existing commercial mapping systems have not been able to consistently and accurately predict the location of the pivot points of the rotor outside of the pulmonary veins.
The aim of this study is to evaluate four recently developed signal processing approaches – multiscale frequency (MSF), Shannon entropy (SE), Kurtosis (Kt), and multiscale entropy (MSE) – in their ability to identify the pivot point of rotors. These proposed techniques utilize different cardiac signal characteristics to uncover the intrinsic complexity of the electrical activity in the rotors, which are not taken into account in current mapping methods. We validated these techniques using high-resolution optical mapping experiments in which direct visualization and identification of rotors in ex-vivo Langendorff-perfused rabbit hearts were possible. Two episodes of ventricular tachycardia were used to evaluate the performance and robustness of MSF, Kt, SE, and MSE approaches with respect to the following simulated clinical limitations: shortened electrogram duration and decreased spatial resolution. To quantitatively assess the performance of the four techniques, results were compared to the “true” rotor(s) identified using optical mapping-based phase maps. The results demonstrate that MSF, Kt, and MSE accurately identified both stationary and meandering rotors. In addition, these techniques remained accurate under simulated clinical limitations: shortened electrogram duration and decreased spatial resolution. These results motivate further validation using intracardiac electrograms to see if these approaches can map rotors in a clinical setting and whether they apply to more complex arrhythmias.
For more information, please visit our group website: http://talkachovalab.umn.edu/