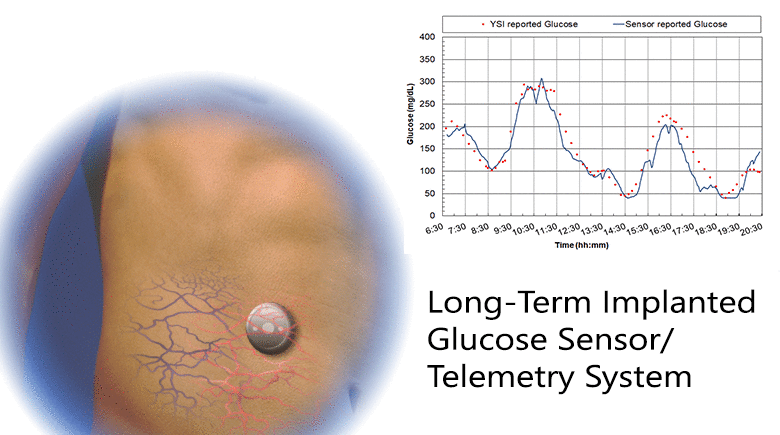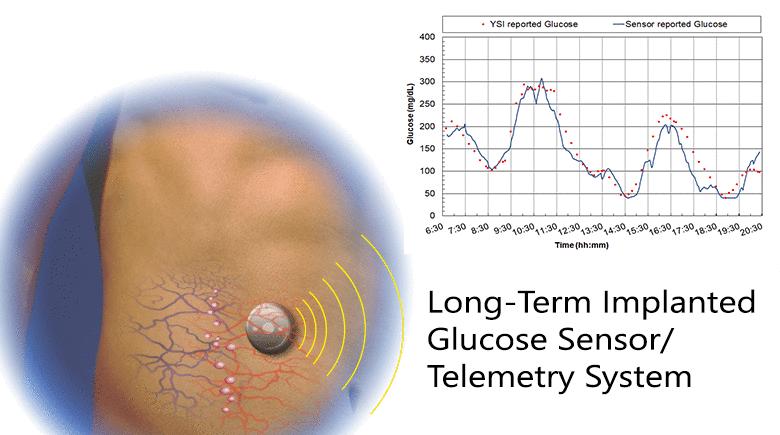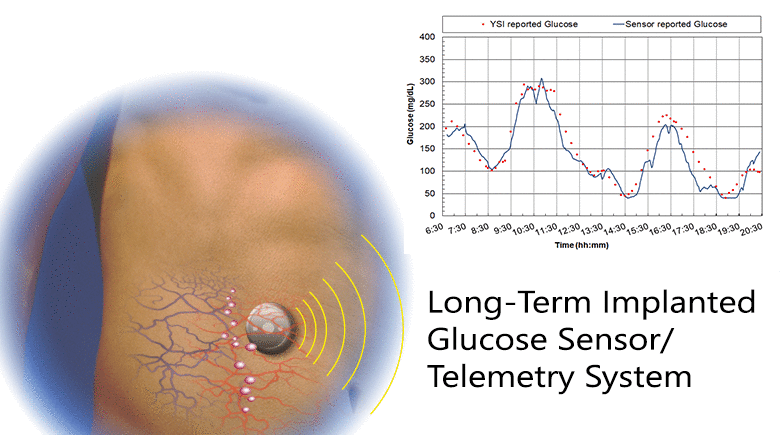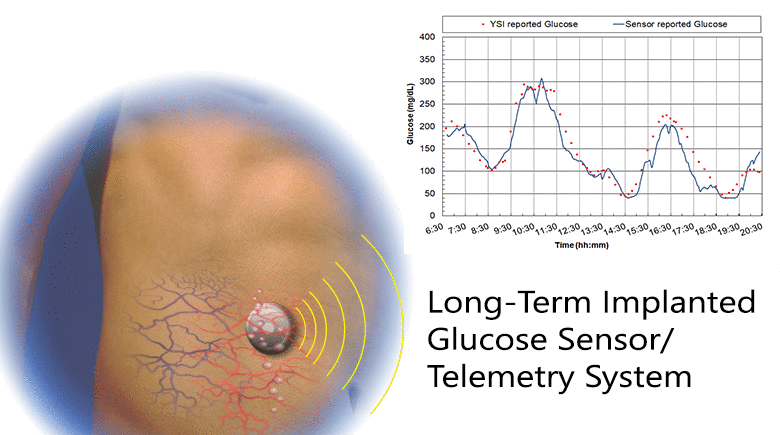
Diabetes is a disease that is growing worldwide. Glucose control is central to all therapies for diabetes and achieving control requires monitoring glucose concentration. We have developed a fully implanted sensor/telemetry system consisting of two electrochemical oxygen sensors, one with a membrane of immobilized glucose oxidase and the other an oxygen reference electrode, and a battery-operated telemetry system that transmits glucose values to an external receiver every two minutes, with the circuitry and battery housed in a hermetically sealed titanium container. We described studies in which sensors were implanted in five individuals with diabetes using a simple outpatient procedure, and operated for up to six months. The studies consisted of recordings of monthly in-clinic glucose excursions compared to reference blood glucose values, and long-term at-home recordings of spontaneous glucose excursions compared to reference finger-stick values. We included a glucose mass transfer model that relates sensor signals to reference blood glucose values, and estimated all model parameters. The goal was to determine if long-term glucose monitoring in humans is feasible with this sensor. Clinical trials are currently underway with a smaller second-generation implant employing the same sensing mechanism and similar design, but having only a fraction of the volume of the present device.
The results: 1) Sensor responses showed a strong association with reference glucose values, as characterized by goodness-of-fit, MARD, and regression coefficients; 2) Sensor calibration varied randomly at an average rate of 2.6%/week, and recalibration was carried out monthly; 3) The permeability to glucose of the tissues adjacent to the implant was comparable to the permeability in solutions; and 4) The results demonstrate the feasibility of long-term, continuous monitoring of glucose in humans. With further development, this system may lead to a new era of glucose monitoring in diabetes.