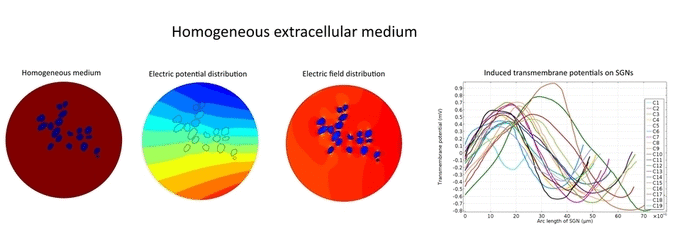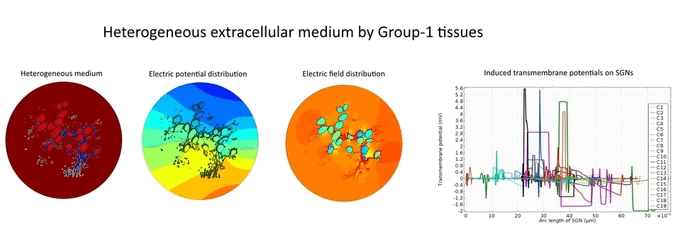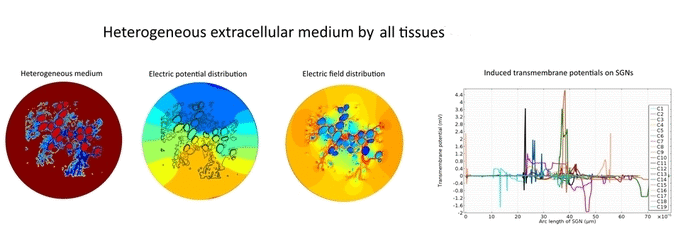
 Electric field applied by cochlear implants stimulates the intact type-1 spiral ganglion neurons (SGNs) in the Rosenthal’s canal of the human cochlea. As a result, transmembrane potential of the SGNs exceeds threshold value and fires the action potential through the auditory nerve which gives the hearing sensation to the profoundly deaf people. However, existing in silico studies could not satisfactorily explain why most of the SGNs in same accommodation and vicinity of the stimulating electrode respond differently to the applied electric field.
Electric field applied by cochlear implants stimulates the intact type-1 spiral ganglion neurons (SGNs) in the Rosenthal’s canal of the human cochlea. As a result, transmembrane potential of the SGNs exceeds threshold value and fires the action potential through the auditory nerve which gives the hearing sensation to the profoundly deaf people. However, existing in silico studies could not satisfactorily explain why most of the SGNs in same accommodation and vicinity of the stimulating electrode respond differently to the applied electric field.
Morphometric studies suggest that type-1 SGNs are surrounded by heterogeneously distributed tissues such as satellite glial cells, Schwan cells, myelinated central axons and type-2 SGNs in the Rosenthal’s canal. In spite of evidential heterogeneity, existing in silico models assumed a homogeneous medium in the Rosenthal’s canal to overcome the modeling complexities.
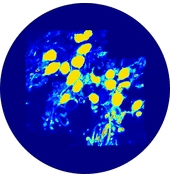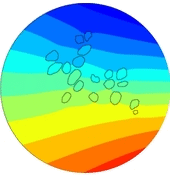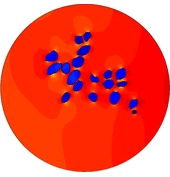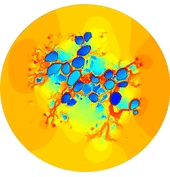
In the present first of its kind simulation study, we considered an image of immunohistochemically stained SGNs in the Rosenthal’s canal. We have implemented the tissue heterogeneity in a two-dimensional finite element model of area of interest. Dielectric properties were assigned based on the intensities of marked tissues in the image and simulated using a bipolar stimulation protocol.
Simulation results suggest that various tissues map different degree of heterogeneity around SGNs in a given accommodation. As a result, applied electric field experiences heterogeneous electric conductivity in the extracellular matrix and induces discrete transmembrane potential on each SGN. This in turn leads to the asymmetric response of SGNs to the applied electric field.
Apart from the academic interest, these results provide useful insights to the latest medical interventions such as drug induced re-sprouting of peripheral axons and gene therapy to treat the profound deafness. These results also play a key role to optimize the cochlear implant functionality.