When it comes to their health, people want answers right now. But clinicians cannot always make snap judgments about ailments or injuries. One way to help both general practitioners and patients is to introduce technologies that deliver quick and accurate diagnoses in a standard clinical setting.
Here, IEEE Pulse features three examples of recently U.S. Food and Drug Administration (FDA)-approved diagnostic approaches that give patients fast responses about their conditions from a simple trip to their doctor, and without the need to see a specialist first. They include:
- an autonomous artificial intelligence (AI) algorithm to diagnose diabetic retinopathy (DR);
- an assay to spot infection with Mycoplasma genitalium, which can cause a sexually transmitted disease (STD);
- an eye-tracking strategy to identify concussion.
AI for DR
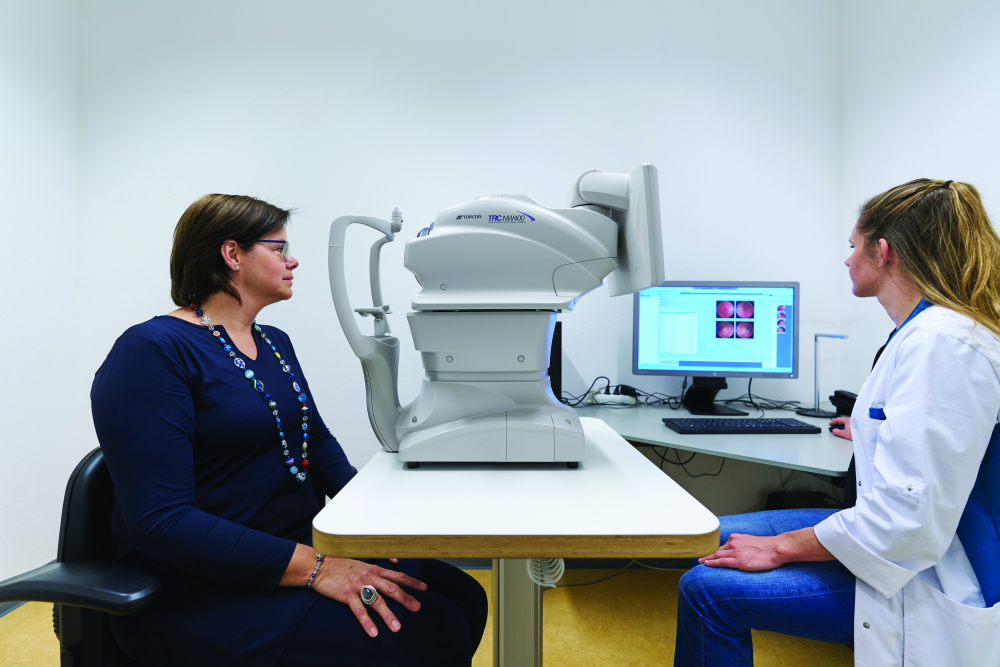
DR is a leading cause of blindness, but it can be treated if caught early. Unfortunately, many patients have little access to care professionals who can diagnose the condition, and those who live in urban areas may find it difficult to get appointments in busy specialists’ offices, said Dr. Michael Abramoff, M.D., Ph.D., professor of ophthalmology and visual sciences, and electrical and computer engineering at the University of Iowa. For more than two decades, he has been putting his expertise in AI and software development to work to find a solution. That effort led to a fully autonomous system capable of making medical decisions (Figures 1 and 2). Abramoff founded and now serves as executive chairman of the company IDx of Coralville, IA, to get that system to market.
Called IDx-DR, the system is an autonomous diagnostic system that Abramoff described as the first application of an AI system that diagnoses disease without human input. It uses a robotic fundus camera, which is a type of special low-power microscope with an attached camera, to capture four images of the back of the eye (Figure 3). The autonomous AI algorithm then scrutinizes them for biomarkers that suggest DR and reports the diagnostic results to the provider managing the patient’s diabetes. Biomarkers include hemorrhages, microaneurysms, exudates (types of cell masses), and intraretinal microvascular abnormalities that are known to be associated with DR, he said. “We wanted to exclusively create detectors that themselves use deep learning to detect different aspects of the biomarkers (that were) aligned with clinical studies.”
The process from the taking of photos through the assessment and diagnosis takes only a few minutes. “The provider sees the diagnosis and discusses with the patient whether the result was good or if there was DR. If it’s good, the patient is just told to come back in three months for their regular diabetes-management checkup. If there is DR, the provider will recommend the patient go see an eye-care provider for treatment and management,” Abramoff said.
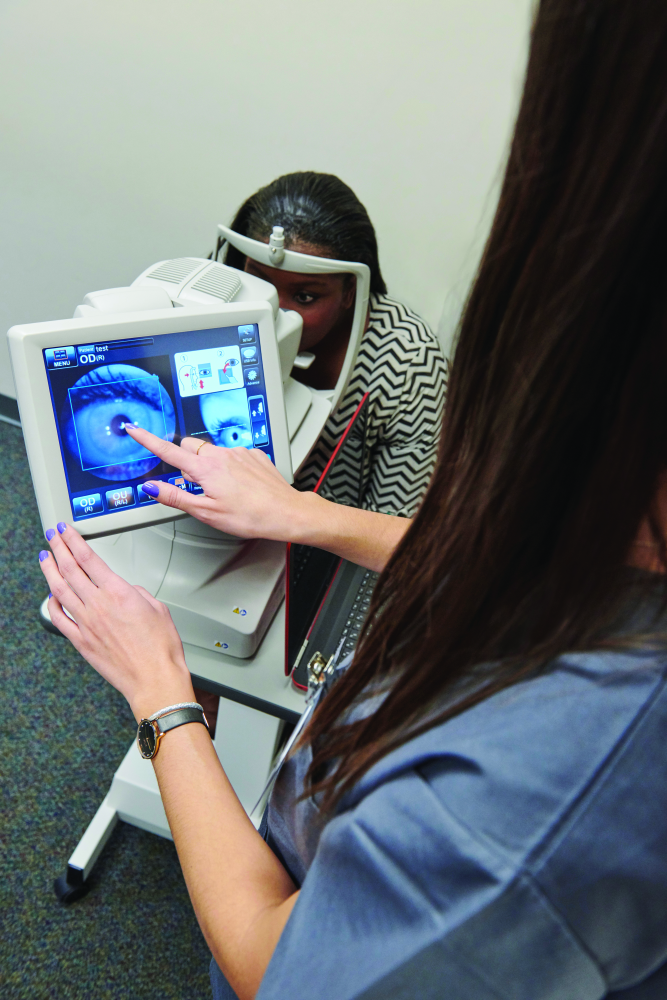
To verify that the system was safe, efficient, and equitable (i.e., absence of racial, ethnic, and other biases) for a wide range of patients, they compared IDx-DR’s results to actual clinical outcomes. He reported, “We exceeded all three superiority endpoints—sensitivity, specificity, and diagnosability—in clinical trials” [1], [2].
With the FDA approval, IDx is marketing the technology for use with people aged 22 and older who have type 1 or 2 diabetes. “It has been used to test patients for more than a year now,” Abramoff said. “In a diabetes education center in New Orleans, some diabetic patients were previously waiting 4–6 months to see an eye-care provider, and so we came in with IDx-DR and now they can make same-day appointments if warranted. Autonomous AI can really solve the patient-access problem.”
The company is continuing to fine-tune IDx-DR, mainly to make it even easier for operators to use. It is also developing additional AI systems to diagnose other diseases, such as macular degeneration, glaucoma, and ear infection, he noted, adding that the company already has a number of prototypes that it hopes to put into clinical trial within the next year.
“Autonomous AI has so much promise in terms of driving down costs, improving quality, and improving access. We need to do more of them, but it’s very important that we do it safely, efficiently, and equitably,” Abramoff said. “I think there’s a risk if we overhype this by glamorizing AI because it’s really cool technologically.” AI needs to gain patient trust, he remarked. “Right now, it’s a crucial period where we need to rigorously validate it, explaining exactly what it’s doing, and be very accountable and transparent about what we’re doing.”
STD wars
Infection with M. genitalium (often shortened to Mgen) is another area that begged for a new diagnostic approach. In fact, no one even knew about this bacterium until 1981, and it took another decade or so before microbiologists recognized that Mgen was behind some of the inexplicable urogenital infections doctors were seeing in both men and women. Mgen drew the attention of the global medical-technology company Hologic, which is headquartered in Marlborough, MA. Hologic and its forerunner company Gen-Probe have been developing assays to identify the major causes of urogenital infections since the 1980s.

For Mgen detection, the company is using its Aptima-format assay that runs on Hologic’s Panther System instrument. “Panther is the automated molecular test system that we use for infectious-disease testing, and these assays use transcription-mediated amplification (TMA), so this is an isothermal, nucleic acid, targeted amplification system that was developed and validated here at Hologic,” said Damon Getman, PhD, the company’s director of research and development. Rather than targeting just one deoxyribonucleic acid (DNA) gene per cell as a DNA polymerase chain reaction (PCR) would, he explained that the Hologic technology isolates 1,000–3,000 copies of ribosomal ribonucleic acid (rRNA) in each cell, and by purifying it and then adding reagents for the isothermal TMA reaction, that rRNA is robustly and quickly amplified. As a result, he asserted, it provides “a sensitivity advantage of several orders of magnitude compared to competing technologies. It’s a very, very sensitive test.”
The Mgen test actually required the development of four TMA assays, including three alternate target assays used to form a composite comparative for the clinical study. “The way this worked is that a patient’s specimen would be tested with the first two of these three alternate assays, and if those two agreed either positive or negative, then that was the final status of the patient: either positively infected or not infected. If those two alternate assays disagreed, we ran the third alternate assay as the tie-breaker, and that then provided the infection status for that specimen,” Getman said, noting that this was the first time such an approach had been used for an FDA clinical validation study [3], [4].
Hologic received FDA clearance for the Mgen test in the spring of 2019, and it is now available commercially to physicians. The biggest challenge at this point is to educate physicians and laboratories who are still unfamiliar with M. genitalium as a potential cause of asymptomatic urogenital infections, which if left untreated, can lead to increased risk for premature labor or low birthweight, and may be associated with cervicitis or pelvic inflammatory disease, Getman said. “We think this test is going to provide a more comprehensive solution for diagnosing patients with these infections, and hopefully provide some clarity about the proper treatment modalities going forward.”
In addition, Hologic is working on tests for antibiotic resistance in M. genitalium and for the gonorrhea bacterium Neisseria gonorrhoeae. “For gonorrhea, there’s really only one drug left that is completely effective for treatment, so resistance testing for gonorrhea is going to be an important area for us in the future as well.”
Eyeing concussion
Concussion diagnosis has been a bit of a guessing game, as it is often based on patients’ self-assessments about how sleepy or nauseous they feel. That isn’t good enough, said Uzma Samadani, M.D., Ph.D., associate professor of bioinformatics and computational biology at the University of Minnesota, and neurosurgeon at CentraCare Health and the Minneapolis VA Medical Center, in St. Cloud and Minneapolis, MN, respectively (Figure 4).

Samadani is also the founder of Oculogica, New York, NY, a company that is commercializing a technology called EyeBox (Figure 5) that takes the guesswork out of concussion. With EyeBox, a patient watches a short video while a camera measures his or her pupil position, and a proprietary algorithm uses that positional information to identify whether the patient may have suffered a concussion, she described.
Samadani was originally introduced to eye-tracking when she was looking for an outcome measure for patients with severe traumatic brain injury, but soon realized that it could reveal when a patient had limitations in their capacity to move their eyes and could therefore detect disruptions of central nervous system (CNS) function, notably concussion. “Systems that assess function tell you how well something is working, such as echocardiogram for the heart, but it doesn’t tell you what’s wrong. The nice thing about eye tracking is it also tells you a little bit about what’s wrong, because you can figure out which pathways are disrupted,” she said. “It’s really quite unbelievable how much you can determine just from looking at people’s eye movements in great detail.”
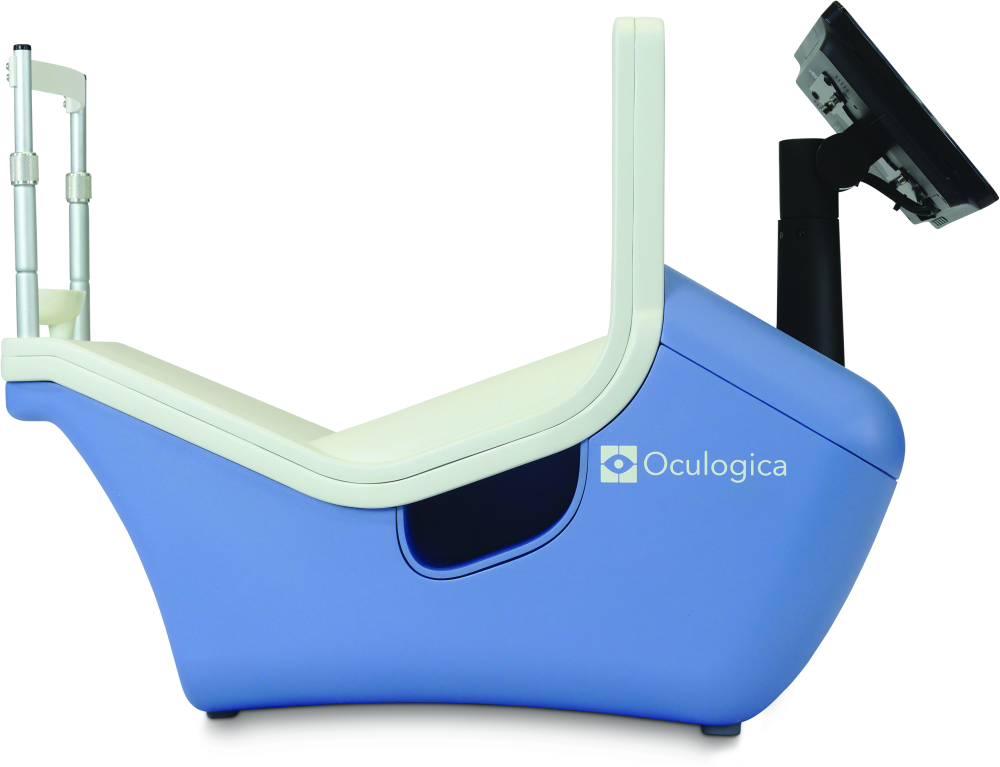
EyeBox works by perceiving subtle anomalies in pupil movements, which Samadani’s research group meticulously recorded in patients with known CNS deficiencies. In an early paper [5], they verified the technology’s ability not only to detect specific cranial nerve palsies, but also to chronicle improvements in pupil movements as the patient responded to treatment. Their work has broadened since then to include a wide range of deficiencies in CNS function. “For many of our eye-tracking metrics, we have correlated them clinically, but we’re also picking up abnormalities that are so subtle that there is no clinical correlate, so there’s no noticeable deficiency,” she said. “For example, skew deviation (nonsymmetric upward movement of the eyes) or convergence insufficiency (inability to smoothly switch from distance to close-up vision) is not necessarily something that you would notice on clinical examination, but we see it with eye tracking because we are recording 500 pupil positions per second, so it’s much more sensitive.”
With FDA approval as well as the American Medical Association’s Current Procedural Terminology (CPT) code, physicians are now able to use—and bill for using—EyeBox, Samadani said, and it is already being employed in a number of hospitals. “Our technology is intended for use in people with suspected brain injury, because it can help classify the nature of their problem,” she said. Oculogica is also investigating eye-tracking technology to test concussion patients for second impact syndrome [6], which can occur when the patient sustains a second hit before the brain has recovered from the first (e.g., still has elevated intracranial pressure), or potentially to screen the general population for such conditions as dyslexia or attention-deficit/hyperactivity disorder (ADHD).
She added, “My primary goal is to change the way we understand the nature of brain injury, and to massively characterize and classify brain injury just like we do kidney, heart, lung, and liver problems. Right now, we’re not able to classify the nature of brain injury at all, and with eye tracking, we can start.”
More to come
IDx-DR, the Hologic’s Mgen test, and EyeBox are just a few of the new diagnostic technologies becoming available. As the health care industry pushes forward on its drive toward greater efficiency and improved outcomes, and clinicians are under continued pressure from patients for immediate assessment, diagnostic technologies are poised to gain even more traction in the medical arena.
Samadani remarked, “I think the future of health care will be increasingly objective and algorithmic both for diagnostics and therapeutics.” She noted, “Mental health in the United States has a long history of being under-recognized and untreated. Objective diagnostics will change this because if you can measure a problem, you can develop a therapeutic for it.”
Technology will indeed have a broad impact on medicine, Getman added. He remarked, “Diagnostic test results are used for approximately 70% of decision-making by health care providers, so new highly accurate devices are key to improving patient care while lowering the costs of that care.”
References
- M. D. Abramoff, P. T. Lavin, M. Birch, N. Shah, and J. C. Folk, “Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices,” npj Digital Med., vol. 1, Aug. 28, 2018, Art. no. 39.
- F. D. Verbraak et al., “Diagnostic accuracy of a device for the automated detection of diabetic retinopathy in a primary care setting,” Diabetes Care, vol. 42, no. 4, pp. 651–656, Apr. 2019.
- B. Kirkconnell et al., “Design and validation of transcription-mediated-amplification nucleic acid amplification tests for Mycoplasma genitalium,” J. Clin. Microbiol., vol. 57, no. 8, p. e00264-19, Aug. 2019. Accessed: February 10, 2020. [Online]. Available: https://jcm.asm.org/content/jcm/57/8/e00264-19.full.pdf
- J. Schachter and M. Chernesky, “Alternate nucleic acid targets can be used to create a composite standard to evaluate clinical performance of nucleic acid amplification tests,” J. Clin. Microbiol., vol. 57, no. 8, p. e00661-19, Aug. 2019. Accessed: February 10, 2020. [Online]. Available: https://jcm.asm.org/content/jcm/57/8/e00661 19.full.pdf
- U. Samadani et al., “Detection of third and sixth cranial nerve palsies with a novel method for eye tracking while watching a short film clip,” J. Neurosurg., vol. 122, no. 3, pp. 707–720, Mar. 2015.
- R. Kolecki et al., “Elevated intracranial pressure and reversible eye-tracking changes detected while viewing a film clip,” J. Neurosurg., vol. 128, no. 3, pp. 811–818, Mar. 2018.



