The Center for Bioengineering Innovation and Design (CBID) at Johns Hopkins University (JHU) has established a comprehensive approach to addressing global health challenges. Central to CBID’s modality on global health is a strategy that integrates education, research, and collaboration. Through its graduate program, CBID trains the next generation of health care innovators to address the specific needs of low- and middle-income countries (LMICs). Graduate student teams at CBID begin their year with a focus on a health care thematic area associated with a target country.
Our team’s thematic area started with a focus on Women’s Health in Uganda. This article reviews the process that we have taken as students trained in global health innovation and discusses some takeaways from the perspective of early-stage innovators. At the time of writing this article, we are now a second-year project. The scope of this discussion includes our beginnings as a student team supported by existing contacts in Uganda and our progression to a grant-funded project with institutional partnerships, a development team working toward evidence generation, pilot studies, and eventual implementation. We hope for these findings to aid the emerging discussion on success factors for impact-centered global health projects, particularly for hopeful innovators entering this uncertain space.
A background on our approach to innovation
With generous initial funding from Hologic (MA, USA) to explore the gaps in health care services in women’s health, we began conducting a landscape analysis of challenges faced by women and their health care providers in Uganda while in our home institution at The Johns Hopkins Whiting School of Engineering (MD, USA). This analysis was informed by a combination of literature review and virtual interviews with Ugandan partners and key opinion leaders (KOLs) in international health. The background research accounted for disease state epidemiology, stakeholder analysis, and technology/existing solutions and their shortcomings. From this landscape analysis, we found priorities in three sub-focuses of sexually transmitted infections (STIs), maternal health, and breast health within the bigger scope of women’s health.
Following this landscape analysis, we traveled to Uganda to conduct ethnographic research within each of the three sub-focuses. We shadowed clinicians in clinic and operating room settings, interviewed patients, community health workers, nurses, researchers, and government officials, and validated assumptions during this in-country immersion using a variety of ethnographic research methods. This immersion resulted in articulating hundreds of unmet clinical needs spanning the three sub-focuses. In addition to the clinical insights gained from our ethnographic research, we created communication channels with researchers and clinical leadership in Uganda that formed the basis of future partnerships.
Subsequently, our team conducted a needs prioritization activity using a thorough four quadrant method of analysis [1, Figure 1]. Factors considered included but were not limited to public health impact, policy alignment, the burden of disease, donor alignment, competitive landscape, and early validation of the technical feasibility of proposed solutions in the area. This process spanned a few months, with further research and validation with in-country stakeholders and interviews with experts at JHU and beyond.
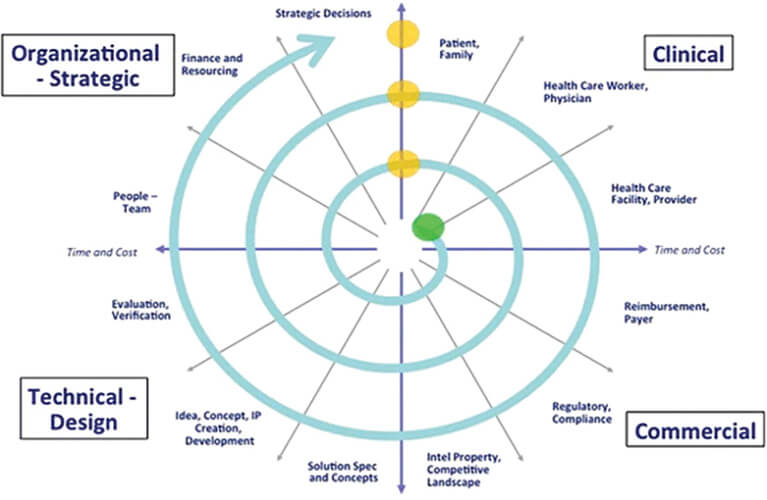
Figure 1. Spiral-iterative innovation model. (Image courtesy of Youseph Yazdi and Soumyadipta Acharya.)
In parallel with this four quadrant analysis, we ideated on potential solutions for the most promising needs. Our team decided unanimously that our top qualifying factor was the level of impact that our work could serve. After evaluating the global solution landscape, we found that challenges within the maternal health and communicable diseases realms are being served by large-scale initiatives like NEST 360 [2] and heavy investments in global HIV and AIDS programs [3]. In line with recent shifts in global health priorities toward women’s health and non-communicable diseases (NCDs), we chose to pursue an innovation target around breast cancer management.
After further needs prioritization and preliminary ideation, we had an opportunity to conduct further ethnography and stakeholder validation in person in Uganda—this time taking a deeper dive into the specific unmet need we were pursuing in breast cancer management. The team targeted this trip around a focused investigation and validation on identifying the end-users, target-use locations, and creating official partnerships with stakeholders to develop the project.
As a result of this work, the team’s innovation target became a focus to increase the availability of breast cancer diagnostics in rural Uganda while increasing the diagnostic throughput of the existing pathologists in the country. The decision to address diagnosis in the breast cancer patient pathway was deliberate—we understood that detection of breast cancer at an early stage was desirable and widely pursued in other global health endeavors, but that population-wide screening was not feasible given cultural barriers and constraints in health care infrastructure [4]. We decided to pursue a method to give the best chances to patients who are already seeking help for breast cancer by providing a timely diagnosis.
As of this article, our team is in the second year of developing the diagnostic venture. Hinging on the success of our development, we will then proceed to usability and pilot studies, engage stakeholders for implementation, and eventually implement and scale our solution for millions of women requiring breast cancer diagnosis in East Africa and beyond.
What we learned outside the textbook
Beyond the process outlined above, our road to eventual implementation was not something that we could follow “by the book.” With some mentorship, we developed our network of stakeholders surrounding breast cancer, wrote our first grants, applied for our first inter-institutional review boards (IRBs), study agreements, and data-sharing agreements, managed fluid stakeholder relationships, wrestled with the impostor syndrome and savior complex, analyzed the acceptability and sustainability of a new solution, amongst other factors not written in many global innovation literature. Even with the backing of a well-resourced and connected institution like Johns Hopkins and an abundance of proficient mentors—the work to create a successful intervention that can potentially help millions of women get an early diagnosis of breast cancer—is sometimes seemingly insurmountable.
Nine out of every ten health technology startups fail [5]. With the current disadvantaged state of investment toward health care innovations catered for the global health community, we are eager for our work to bring about real value to women of disadvantaged conditions suffering from breast cancer. In the following paragraphs, we share a few highlights of our experiences as we navigate breast cancer innovation in low-resource regions.
Grounding ourselves from a place of privilege
It was not easy to know how to feel the first time one of our local guides, a Ph.D. candidate and researcher in public health, brought a discomforting topic to our lunch table. He said something along the lines of “If you do not bring about impact to patients in hospitals here, what you are doing is tourism. The money spent on this trip could be spent buying useful equipment in hospitals.”
As global health innovators, we know that time and capital are invested in investigating potentially life-changing solutions for many more people—by making investments “driven by social return,” as USAID does with the development innovation ventures (DIV) grants [6]. In economic terms, advancements in technology can push the boundaries of capacity for real growth by shifting the production-possibility frontier (PPF). These are concepts that make sense to us as outsiders, where outcomes are evaluated in numbers and statistics.
While our intentions are mission-driven, it is important to understand the realities on the ground. Every dollar spent on innovation for tomorrow could be spent making improvements today. Working in global health innovation is a privilege. We must truly internalize this perspective and ensure that the resources channeled through such programs achieve the intended impacts in a cost-effective and timely manner.
Find the champions
Working with a local stakeholder with a passion for the problem, strong expertise, and connections can help open many doors. Dr. Robert Lukande, a senior lecturer and pathologist at Makerere School of Medicine is one such champion. His profound expertise and deep-rooted connections within the health care community have been instrumental in understanding the nuances of clinical challenges that extend beyond the health care system’s surface. Such partnerships enrich our understanding of the health care ecosystem, encompassing interactions among health care workers, services, equipment, and patients.
The endorsement and support from local pathologists, who are among the most dedicated yet overburdened professionals in Uganda, underscore the critical role of in-country champions [7]. These pathologists are not just medical experts; they are advocates for patient care, eager to ensure accurate diagnoses. Our engagement with them helps us tailor solutions to meet real needs. For instance, insights from Mbarara Regional Referral Hospital revealed unique cytology staining techniques, prompting us to adapt our approach from the onset.
We have been lucky enough to engage with several such champions. Figure 2 shows some of our partners at the Uganda National Health Laboratories and Diagnostic Services (UNHLDS). They help maintain our engagement with local key stakeholders and help us work through some local bureaucratic procedures that we are unfamiliar with. They are also great mentors who have guided us with best practices in interacting with clinical stakeholders and patients, resource procurement, and adhering to cultural norms and legal requirements. Such in-depth local collaboration has been essential in crafting solutions that are not only innovative but also culturally sensitive and legally compliant and are indispensable to working in a global health setting.

Figure 2. We found our champions in the National Health Labs who have been supporting us with data collection. (Photo courtesy Krishna Tejaswini Sathi.)
Global health innovation is a long game
Efforts toward sustainability: co-development of solutions through partnerships
Our project’s approach to understanding hospital capacities was not only aimed at identifying gaps but also at forging partnerships and laying the groundwork for public health implementation support. The collaboration with Makerere University’s School of Public Health (MakSPH) and other stakeholders underscored the project’s commitment to developing a solution that is not only technologically innovative but also culturally sensitive and practical to scale within the existing Ugandan health care context. This comprehensive assessment strategy ensured that the project’s implementation plan would be well-informed, feasible, and tailored to the specific needs and capacities of the target hospitals in Uganda.
Through our partnerships, we will test and iterate through the solution with our partners and eventual users by conducting multiple pilot and usability studies to inform how our technology can best be built out to support breast cancer diagnosis. Figure 3 captures one early feasibility study conducted with our study partners.

Figure 3. Engaging with our partners in Mulago Hospital, National Health Laboratories (NHLDS), and Mbarara University of Science and Technology. (Photo courtesy Dan Niwaha.)
Efforts toward sustainability: a local-centric business model
An effective solution must also be self-sustainable. Programs that rely solely on donor funding can only last till the end of their funding cycle. With a focus on creating a sustainable impact, we understand that more than innovation in technology itself is needed and that there needs to be a well-thought-out business model and implementation.
In line with WHO’s Sustainable Financing for Health accelerator, we are working toward a country-led, demand-driven, and evidence-informed method for implementation, leveraging the existing multitude of development partnerships in the intersection of innovation, global health, and MedTech industry from an early developmental stage [8].
It is important to plan with sustainability in mind from an early stage as it would inform the design and implementation of the solution and avoid the creation of a product that will only sit on the prototyping shelves without reaching the intended users.
Statistically speaking, 90% of health technology startups fail [5] in the long term, and 60% fail within five years. Low-resource economies receive disproportionally low attention and resources in health care innovation compared to their high-income counterparts (only 0.2% of all grant funding to NCDs opportunities are allocated to LMICs). We cannot rely on technologies from developed economies to adequately support the needs of LMICs [9]. As one of our mentors, Dr. Harshad Sanghvi, shared, “In global health, we cannot afford to fail at the rate that medical technology startups are failing.” We hope that our learnings will contribute to the growing literature body that will inform and support the success of efforts in global innovations with sustainable impact toward health equity and health care outcomes.
References
- Y. Yazdi and S. Acharya, “A new model for graduate education and innovation in medical technology,” Ann. Biomed. Eng., vol. 41, no. 9, pp. 1822–1833, 2013, doi: 10.1007/s10439-013-0869-4.
- NEST360. (Jan. 26, 2024). Nest360 Technology. [Online]. Available: https://nest360.org/technology/
- S. Luboga et al., “Did PEPFAR investments result in health system strengthening? A retrospective longitudinal study measuring non-HIV health service utilization at the district level,” Health Policy Planning, vol. 31, no. 7, pp. 897–909, 2016, doi: 10.1093/heapol/czw009.
- E. Black and R. Richmond, “Improving early detection of breast cancer in sub-Saharan Africa: Why mammography may not be the way forward,” Globalization Health, vol. 15, no. 1, p. 3, 2019, doi: 10.1186/s12992-018-0446-6.
- I. Chakraborty, S. Edirippulige, and P. V. Ilavarasan, “What is coming next in health technology startups? Some insights and practice guidelines,” Digit. Health, vol. 9, Jan. 2023, Art. no. 20552076231178435, doi: 10.1177/20552076231178435.
- U.S. Global Develop. Lab, U.S. Agency Int. Develop. (Apr. 20, 2020). Development Innovation Ventures. [Online]. Available: https://www.usaid.gov/div
- Seed Global Health. (Apr. 22, 2019). South-South Collaboration: Transforming Pathology Training and Practice in Uganda. Accessed: Feb. 15, 2024. [Online]. Available: https://seedglobalhealth.org/2019/04/22/transforming-pathology-training-and-practice-in-uganda/
- The Global Action Plan for Healthy Lives and Well-being for All—Accelerator. Accessed: Feb. 15, 2024. [Online]. Available: https://www.who.int/initiatives/sdg3-global-action-plan/accelerator-discussion-frames
- (2023). Global Observatory on Health R&D: Bridging the Gap in Global Health Research and Development. [Online]. Available: https://www.who.int/news/item/21-11-2023-global-observatory-on-health-r-d–bridging-the-gap-in-global-health-research-and-development



