Depression and chronic pain know no geographical boundaries. About 350 million people around the world experience long-lasting sadness and an unshakeable sense of hopelessness, and one person out of ten tries to live each day to its fullest despite continuous physical pain. These two difficult conditions frequently coexist, becoming more common with age. Looking ahead, we can expect the incidence of depression and chronic illness to grow, since more people over age 65 will populate the world by 2020 than children younger than five.
Amid an array of pain-management therapies and mood-altering therapeutics, researchers are looking at technologies focused on patients who are immune to drug and psychotherapy interventions. Among them, deep brain stimulation (DBS) has been found to provide much-needed relief for those experiencing treatment-resistant depression and refractory chronic pain. This article investigates new frontiers of DBS in depression and pain management in the context of a historical review and current market analysis of this platform technology.
DBS for Depression and Chronic Pain
To date, DBS represents an advanced surgical procedure to treat motor deficiencies in a growing scope of neurological illnesses that includes Parkinson’s disease, essential tremor, and dystonia. Using variable electric impulses sent to specific brain regions, DBS amplifies or suppresses neural circuits to reverse pathology. While DBS intervention is considered to be young—having been clinically incorporated for only 16 years—its encouraging results in patient health outcomes have garnered much attention and raised hopes for the treatment of traditionally untreatable conditions.
Interestingly, advancements in DBS have ignited curiosity about its efficacy in treating behavioral maladies and psychopathologies that might not otherwise be treated by drug therapy. Specifically, it has been shown to effectively minimize symptoms of intractable obsessive-compulsive disorder (OCD) and major depressive disorder (MDD).
![The epidemiology of depression and chronic pain, global statistics. [Graphics courtesy of the Alfred E. Mann Institute for Biomedical Engineering at the University of Southern California (AMI USC), 2014.]](https://www.embs.org/wp-content/uploads/2015/03/gosset01-2386511.png)
Americans are more depressed than Europeans and four times more than Australians. With a lifetime prevalence of depression of 15% in the United States and 9% in Europe, MDD or depression is a complex mental illness marked by depressed mood or severely diminished interest in all activities along with a host of other factors, which result in functional impairment, including insomnia, significant weight change, psychomotor agitation or retardation, fatigue, and recurrent suicidal ideation. Further, depressive mood disorders are becoming increasingly related to comorbid medical conditions and lowered immune function, which contribute to the World Health Organization’s recognition of depression as the leading cause of disability worldwide (above). Although antidepressant drug therapy is often effective, up to 30% of those with depression are resistant to traditional forms of rehabilitation, including electroconvulsive therapy and psychotherapy. For patients with refractory or treatment-resistant depression, DBS may provide a viable solution to an otherwise impervious illness.
Another potential use of DBS—unapproved by the U.S. Food and Drug Administration (FDA)—is for the treatment of chronic pain conditions. According to a recent poll conducted by the American Pain Society, 30.7% of adults in the United States experience some form of chronic pain for at least a six-month period. Additionally, the World Health Organization reports that 22% of primary care patients struggle with persistent pain conditions, where pain is the most common cause of medical attention. Despite their prevalence, a wide range of neuropathic and nociceptive pain conditions are not sufficiently relieved through surgical or pharmaceutical intervention. Moreover, pain due to injury, stroke, back, or spine pathology and other persistent aches can result from complex pathways that are not presently treatable. A significant market for DBS may also be found in alleviating chronic pain in individuals who suffer from refractory pain states.
As a growing market for this technology develops, new indications for treatment have been proposed to further expand the scope of use while also providing unprecedented alternatives of improved health for certain patient populations. Among those who suffer from MDD, a leading cause of disability worldwide, treatment-resistant depression is a particularly debilitating illness immune to drug and psychotherapy interventions. Similarly, intractable chronic pain conditions cause significant impairment of daily function with limited treatment options.
Biological Mechanisms
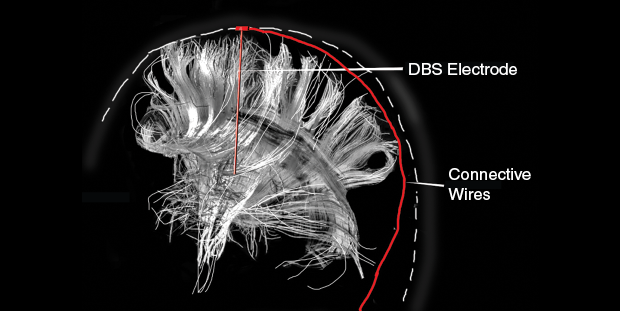
Clinical application of DBS targets specific subcortical brain regions that are believed to be the site of dysfunctional neural activity. Procedural execution for Parkinson’s disease, for example, directs electrode implantation into the subthalamic nucleus or globus pallidus due to abnormal neuronal synchrony related to the motor circuit (above). Electrical stimulation in one of these regions has been shown to correct tremor and dyskinesia for patients with Parkinson’s disease. For depression, several regions have been identified that may contribute to its pathology. The most commonly targeted brain region for DBS in clinical trials has been the subgenual cingulate gyrus (SCN), also known as Brodmann area 25, as it has been shown to yield heightened metabolic activity during depression (below). The SCN is also a key facet of the limbic-cortical system, which contributes to an integrated neural network responsible for mood state that may result from multiple interacting brain regions. Several studies corroborate the effectiveness of DBS targeting the white matter associated with the SCN to confer sustained long-term relief of depressive symptoms. Another area targeted for refractory depression has been the nucleus accumbens (NAcc) that is closely related to reward systems of the brain that drive behavior. DBS targeting the NAcc—and its accompanying system of the medial forebrain bundle—has also been demonstrated to reduce depressive symptoms as soon as days after intervention and result in full remission of MDD months after the procedure. While the precise mechanisms of DBS are still not fully understood, it appears that electrical stimulation may realign pathological molecular and chemical imbalances to restore normal functioning.
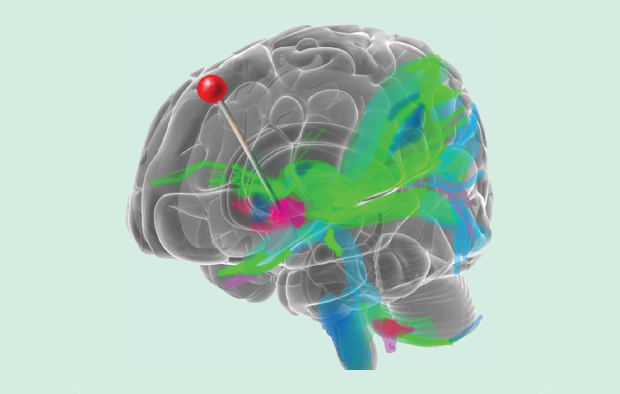
DBS has also been proven to have long-lasting effects to treat certain cases of refractory chronic pain. Most research to date has focused on two areas, depending on the nature of the pain condition: the periaqueductal gray (PAG) and the sensory thalamus (ST). In a meta-analysis conducted by Bittar et al., the long-term efficacy of pain alleviation of a variety of pain conditions was highest in stimulation of the PAG (79%) when compared to stimulation of the ST (58%). The greatest improvement was seen in simultaneous stimulation of the PAG and ST at 87%. Additionally, DBS was found to be more effective in sustained relief of nociceptive pain as opposed to neuropathic pain states and nonresponsive to certain pain states, including paraplegia and anesthesia dolorosa. However, DBS meets many chronic pain states with success, including stroke, amputation rehabilitation, multiple sclerosis, and spine injury, with improved emotional well-being and quality of life.
History and Regulatory Conditions
DBS technology was first experimentally used for the treatment of chronic pain in the 1960s. Much of its initial appeal during that time has been attributed to the success of cardiac pacemakers, which helped to legitimize the use of electrical stimulation for clinical interventions. In fact, the DBS device blueprint closely matches and evolved from traditional cardiac pacemaker units—the first prototyped models of neurostimulators were, indeed, fashioned from the circuit boards of pacemakers. While Medtronic pioneered the effort of neurostimulatory devices in chronic pain, the first FDA approval of DBS was for treatment of essential tremor in 1997. Subsequent additions to approval of DBS for treatable conditions under FDA regulation were Parkinson’s disease in 2002 and dystonia in 2003.
Due to the inherent risk associated with the DBS procedure, regulatory approval has been limited to conditions in which the intervention is seen as a last resort when all other traditional forms of treatment have failed. Perhaps the more apparent reductions in quality of life for movement disorders like Parkinson’s disease and dystonia have been a contributing factor for their approval in recent years. While no FDA clearance has since been granted for the use of DBS for psychiatric conditions or chronic pain, allowance has been made for intractable OCD. In 2009, the FDA approved the Medtronic Reclaim DBS device to treat refractory and drug-resistant forms of OCD under the humanitarian device use exemption (HDE). The HDE FDA regulation recognizes medical technologies that treat a small and underserved population of patients and is similar in form to a premarket approval with fewer regulatory hurdles prior to acceptance.
Market Analysis and Reimbursement
One of the attractive and unique qualities of DBS technology is its potential universality to treat a broad range of conditions. Because of its interventional modality in the brain, it represents a platform technology, wherein minor modifications to the device or lead placement can be adapted to affect a wide range of behavioral and neurological changes. Considering the prevalence of depression (15%) in the United States and the many chronic pain states that arise from injury and disease, the market for DBS could see a significant expansion. It should be noted that high variability is present on a case-by-case basis in the decision and efficacy of treating refractory pain states and depressive illness. Thus, it would be difficult to predict what percentage would be eligible for the intervention from current regulatory standards. What is clear, however, is the predicted rise in need for DBS within its current framework of treatable conditions. The prevalence of Parkinson’s disease, for example, is expected to rise for those over 50 years old, to the extent that the approximately 4.5 million afflicted in 2005 will double by 2030 to 9 million.
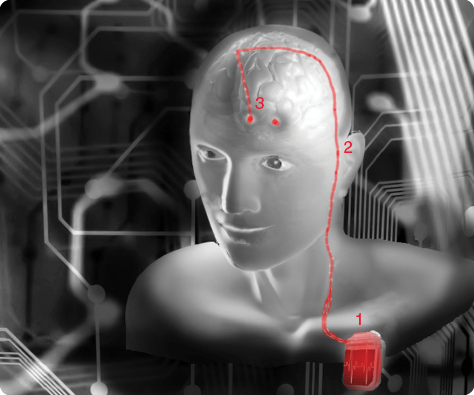
The procedure for DBS is often defined by two separate surgeries to 1) implant the electrodes and 2) insert the generator (below). First, the electrodes are inserted through the skull either unilaterally or bilaterally, where two incisions would be made—both options have separate billing codes for reimbursement. The average cost for unilateral insertion of DBS is between US$35,000 and US$50,000 and can be doubled for concurrent bilateral electrode implantation. Most insurance companies, however, do cover the costs for FDA-cleared DBS devices. The insertion of the generator or battery pack is usually placed under the clavicle and requires a second inpatient procedure for which there are supplementary current procedural terminology codes. Subsequent procedures may include battery replacement and electrode lead removal.
Current Business Models
Opening the use of DBS to a new set of psychiatric applications will benefit from the clinical validations Medtronic and Boston Scientific carved for the treatment of Parkinson’s disease. The first device that received FDA clearance—and perhaps the most widely used clinically—is the Medtronic model 3389. Medtronic was even credited with coining the term “deep brain stimulation” after opening up a neurosciences division in 1975 to investigate the technology. Currently, Medtronic has four neurostimulators available, all of which treat Parkinson’s disease and essential tremor, and three of the four are also used for dystonia (see Table 1). Boston Scientific produces a single DBS device called the Vercise, which is advertised as having the smallest stimulator footprint and boasts a multiple independent current control for precise treatment.
[accordion title=”Table 1. A Current Product List of DBS Technologies, Reporting Treatment Options, Battery Life, Channel Capacity, and FDA and CE Mark Approvals for Prominent Company Models.”]
PD: Parkinson’s Disease; ET: Essential Tremor; D: Dystonia
| Distributor | Model | Channels | Treatments | FDA Approval | Battery Life (Years) |
| Medtronic | 37601 | Two | PD, ET, D | Yes | 9 |
| Medtronic | 37612 | Two | PD, ET | Yes | 9 |
| Medtronic | 37602 | One | PD, ET, D | Yes | 9 |
| Medtronic | 37603 | One | PD, ET, D | Yes | 9 |
| Boston Scientific | Vercise | Two | PD, D | Yes | 25 |
| St. Jude Medical | Libra | One | PD, D | No | 10 |
| St. Jude Medical | Brio | Two | PD, D | No | 10 |
| St. Jude Medical | Libra XP | Two | PD, D | No | 10 |
[/accordion]
In recent years, the DBS market has seen promising growth. Between 2012 and 2013, the DBS market grew 6.7%, with sales revenues for 2013 reaching a record US$677 million. The procedure has proven its efficacy for the treatment of neurological disorders as the adoption rate will steadily increase, with 7–9% annual growth forecasted. Kalorama Information reports an estimated US$4–5 billion annual market for electrical stimulation devices in treating pharmacoresistant depression alone. Some calculations even predict that DBS will reach US$1 billion in revenues by 2018.
Considering the portfolio of similar medical stimulation systems, DBS has outperformed its competition. Medtronic’s Restorative Therapies Group includes the manufacture of surgical technologies, neuromodulation and spine stimulation systems. In 2013, this suite of therapy technologies performed well due to the outstanding success of the DBS product revenue, which balanced the decline in sales of spine stimulation systems that now compete with morphogenetic protein and balloon kyphoplasty. Medtronic was estimated to own roughly 40% of the global DBS market in 2012.
Every unique clinical indication for the use of DBS requires separate FDA approval. Despite prior FDA clearance for the use of DBS to treat Parkinson’s disease, for example, new standards of approval are necessary before psychiatric applications are pursued. Several business strategies can accelerate the approval process for DBS technology for the treatment of psychiatric conditions if the disease selected affects a small population in the United States. The FDA approved the use of the Medtronic Reclaim DBS device for the treatment of OCD in 2009 under the provision of HDE—targeting a condition affecting fewer than 4,000 Americans per year. An HDE application includes similar requirements to a premarket approval application but does not have the rigorous standards for device clearance that large clinical trials require. An applicant seeking the HDE consideration must provide evidence of the rarity of the clinical condition and the absence of an appropriate treatment for the condition. This approach has been effective for Medtronic and pursued again for receiving approval for the treatment of chronic, intractable primary dystonia.
St. Jude Medical pursued another effective business approach to speeding up the market availability of the Libra and Brio neurostimulator DBS systems for the treatment of Parkinson’s disease. Both products were first launched in Europe, followed by later approval to sell in the United States. Libra and Brio obtained the CE Mark in 2009, which created a channel of revenues from European sales that funded clinical trials in the United States for FDA approval.
Final Thoughts
Successfully ameliorating chronic and debilitating disease states, DBS has emerged as a platform technology with unparalleled potential across a number of medical fields to reverse intractable pathology. Regulatory clearance of DBS for certain motor-related diseases has shown promising results in patient health outcomes, and yet further investigation may lead to an approved expansion of treatable conditions to include psychiatric illnesses such as MDD and relief from refractory chronic pain.
Progression of DBS may also benefit from a recent push in federal funding in the neurosciences. In 2013, the Human BRAIN Project (Brain Research Advancement through Innovative Neurotechnologies), funded by the U.S. National Institutes of Health, was announced to represent a large-scale effort to better understand the pathways of brain function and autonomic regulation. For the fiscal year 2014, the Human BRAIN Project received presidential support with a pledge of US$100 million in funding—and determination of “high priority”—for progressing innovative technologies for brain imaging and neuromodulation. Additionally, the U.S. Defense Advanced Research Projects Agency has launched a US$70 million plan over the next four years specifically to develop and enhance DBS technology. A hopeful future awaits the investigation of neuroscience as an imminent convergence of knowledge, technology, and clinical application may maximize the potential of DBS.
For More Information
- T. E. Schlaepfer, B. H. Bewernick, S. Kayser, B. Madler, and V. A. Coenen, “Rapid effects of deep brain stimulation for treatment-resistant major depression,” Biol. Psychiatry, vol. 73, no. 12, pp. 1204–1212, 2013.
- A. M. Gray, E. Pounds-Cornish, F. J. Eccles, T. Z. Aziz, A. L. Green, and R. B. Scott, “Deep brain stimulation as a treatment for neuropathic pain: a longitudinal study addressing neuropsychological outcomes,” J. Pain, vol. 15, no. 3, pp. 283–292, 2014.
- E. A. Pereira, A. L. Green, and T. Z. Aziz, “Deep brain stimulation for pain,” in Brain Stimulation (Handbook of Clinical Neurology, vol. 116). Amsterdam, The Netherlands: Elsevier, 2013, pp. 277–294.