From Ant-Man to the Incredible Shrinking Machine, society has long envisioned developing devices tiny enough to enter human cells. Such nanotechnology could revolutionize the diagnosis of diseases like cancer and neurodegeneration, span new methods of precise drug delivery, and even directly repair damaged organs.
Nanomaterials are already used in a host of products such as sunscreens, food, and cosmetics, but equipping these tiny particles with more active functions— the dream of nanomedicine—is still, for the most part, distant. Although researchers in academia and industry alike have pursued developing nanorobotics in medicine, shrinking any hardware runs into its fair share of problems.
The Size of the Nanoscale
A DNA molecule is 2.5 nm; proteins 10 nm; virus 100 nm;
bacterium 1000 nm; human cells 10,000 nm.
A major challenge seems simple on the normal scale: movement. But on the nanoscale, no battery is small enough to power a nanobot. Teams across the globe are exploring different options to control nanorobotics in the body, with approaches ranging from using electromagnetic and chemical tactics, to tapping into nature.
The second challenge is the body itself. To effectively perform a function, nanorobotics must evade the array of defenses the body employs against tiny intruders, surpassing or evading natural barriers (like the blood–brain barrier) and withstanding sometimes harsh environments, like an acidic human stomach or T-cell-filled bloodstream. What’s more, any nanomaterials used in the body would need to be evaluated for toxicity and prevent against an unwanted immune response.
Furthermore, the science itself is still being understood. One nanometer is 100,000 times less than the diameter of a hair and, when you shrink things down to that size, properties of materials are fundamentally different. Forces that normally don’t have to be taken into account—such as the influence of nearby molecules—now do. Despite these challenges, the field, though still in its nascent phase, is making progress in these areas, and researchers are optimistic about the potential for nanorobotics to revolutionize targeted medicine, particularly cancer.
Nature as Inspiration
Most nanotechnology for medicine entails using small particles to carry materials and deliver them to or within cells. Often this delivery happens by chance; controlled delivery efforts, however, aim to develop very simple robotic systems made up of a payload and shell that can be directed to a specific site. These devices are guided by external forces—such as electromagnetic fields—or through fabrication techniques that take advantage of chemical or biological reactions.

“The topic of nanomedicine as containers that can deliver pharmaceutically active compounds in a targeted way has been around a long time,” says Peer Fischer, a professor of physical chemistry at the University of Stuttgart who heads the independent Micro Nano and Molecular Systems Lab at the Max Planck Institute for Intelligent Systems in Stuttgart (Figure 1, right). “What is tough to do, and what many groups are focusing on, is making these carriers active. For example, rather than relying on a passive diffusion process to distribute a medicine in the blood, a controlled swarm of nanoparticles could be sent to difficult-to-reach areas, like a tumor.”
When it comes to generating motion, brute-force mechanics aren’t a good approach on the nanoscale, because it’s difficult to generate that force locally. What’s more, nanorobotics aimed at entering organs like the lung, intestine, stomach, or eyes, for example, have to deal with one of the body’s main defenses against micro-intruders: complex biological fluids like mucus.
In a one–two punch to tackle the problem of locomotion and entering through mucus, Fischer’s group looked to the ulcer-causing helio pylori bacteria for inspiration, both for its corkscrew-like shape as well as for its ability to discrete enzymes and disrupt the mucus. The bacteria’s small size and shape allows it to move through soft tissues and fluids like mucus, where motions like drilling (rather than swimming) are needed to propel. This is because, as one goes smaller, what previously appeared as a homogeneous liquid distinguishes into a more “spaghetti-like” molecular mesh of macromolecules, explains Fischer. If the diameter of the propeller is small enough, it can drill through the gaps in this network.
“That’s how very small systems can actively move through these penetration barriers with very little force,” Fischer says. “Bacterium is literally a drill.”
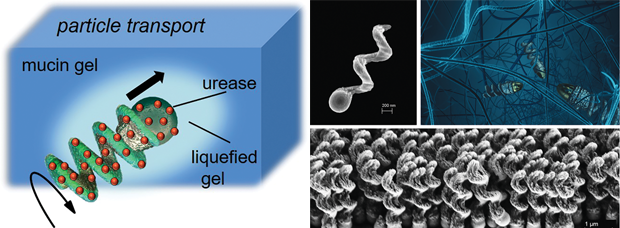
Fischer’s group mimics the corkscrew shape of the pathogen by using a custom 3D fabrication manufacturing process to create small propellers roughly 400 nm long (about 20 times smaller than a human blood cell) made out of silicon dioxide and other materials. These propellers make a similar corkscrew motion through fluid and can be controlled magnetically (Figure 2). A collaborative group led by the Max Planck Institute for Intelligent Systems reported on using the technique to design slippery nanopropellers that successfully drilled through a dissected pig’s eye without damaging tissue, showing the possibility of using the devices for precise eye surgery [1].
In addition to propellers, the team’s lithography and fabrication techniques lets them create billions of nanorobots in several hours made of different materials to impart different functions.
That’s where the next bacteria-inspired idea comes in: actuating microparticles by giving them a chemical source of energy rather than mechanical or magnetic. This chemical engine would comprise half of a nanoparticle containing chemicals that, on release, create an imbalance of concentration gradients, inducing flows to propel a particle in a direction. This would be advantageous over a magnetic control system, says Fischer, because it would allow autonomous operation.
“We want to tailor these chemical reactions in a way to make not only the particle move, but also respond to external signals and gradients,” says Fischer. “In principle this would be a very elegant way to have nanosystems that can locomote and navigate without external control.
A research group in Canada is also inspired by bacteria: Sylvain Martel, director of the Polytechnique Montreal Nanorobotics Laboratory, has worked for decades on coupling living, swimming bacteria to microscopic magnetic beads to create hybrid devices that can be steered by MRI, for example. The bacteria self-propel due to their tails (flagella), and the magnets direct them where to go.
These self-propelled and controllable hybrid “nanobots” could potentially be used as a way to target hard-to-reach tumors. As detailed in [2], Martel and collaborators showed how a swarm of these devices—made up of millions of bacteria combined with sensors and magnetic beads—could reach tumors in mouse models. Martel is optimistic about this approach to treat tumors that traditional chemotherapy often can’t reach.
“Presently, therapeutics are delivered systematically, which cause severe toxicities for the patient while limiting the amount of therapeutics reaching solid tumors,” says Martel. “A robotic approach could help achieve such a goal and for that a wider interdisciplinary approach involving biomedical engineers that were quasi-absent so far in this particular field may help counteract many of the major obstacles facing drug delivery in cancer therapy and where nanotechnology plays a great role.”
The World’s Smallest Medical Robot
Size is critical when it comes to devising new techniques to get into cells and precisely target medicine in additional efforts. Bacteria range around 1000 nm (1 micron), far too large to enter cells or tiny blood vessels.
So far, a device created by University of Texas at San Antonio Professors Ruyan Guo and Amar Bhalla, together with their doctoral student Soutik Betal, holds the Guinness Book of World Records title for smallest medical robot in 2018. Their 120-nm robot can push miniscule payloads and enter the membranes of cells, abilities that they hope will make it useful for medical applications.
“One feature we documented is the ability to transport cells from one position to another. The second feature is the ability to penetrate a cell membrane, difficult for most nanoparticles,” says Bhalla.
“Normal nanocomposites are bigger than the channel of a cell membrane so can’t easily enter a cell,” adds Guo. “But because of our particle’s size and field-driven rotation and torque function, we can direct our nanorobots to penetrate the cell membrane, which would allow you to directly release a payload to destroy cancerous cells, for example.”
One-thousandth the width of a human hair, the team’s nanorobot is made up of a core of cobalt ferrite (essentially a magnetic crystal, possessing its own north and south poles) and a shell consisting of barium titanate (a ferroelectric composition that can generate an electric charge/potential in response to stress). This composition means, very generally, that if a magnetic field is applied, the particle will try to rotate to align itself with the field, with its polarization rotating accordingly.
“This movement enables the work, which gives the nanorobot a force by which you can push objects around or carry something over a certain distance,” says Guo. Their composite, she adds, enabled them a more precise way of controlling direction than others who have used an external electromagnetic approach to manipulating nanomachines, as she and colleagues detailed in [3].
The team, which is developing partnerships and has a patent pending for the technology, next plans to continue understanding design principles to refine the control interactions with cell membranes. They aim to ultimately target not just cancer but also blood clots and maybe someday repair brain cells affected by Alzheimer’s. In addition to medical uses, they are also exploring the possibility of using their nanoparticles in the field of communications.
Bridging Biology and Engineering

Aside from electromagnetic efforts, others are using biology itself to develop and control nanoparticles.
Yang (Claire) Zeng, PhD/MD and research fellow at the Shih Lab at the Department of Cancer Biology at the Dana Farber Cancer Institute/Harvard Medical School, combines studies of nanoscale materials with cancer immunotherapy, focusing on nanodevices made up of folds of DNA to hold payloads— essentially organic nanobots (Figure 3, right).
The origami technique, pioneered by Paul Rothemund at the California Institute of Technology, generally entails folding long strands of scaffold DNA with smaller strands that act like staples to form into 2D or 3D shapes. The Shih Lab has since built upon the approach and made headlines for using the DNA origami method to develop different shapes of 3D DNA nanoparticles that can hold payloads—essentially DNA nanorobots aimed at medical applications like cancer and HIV. The devices consist of hundreds of 40–50 base pairs short single-strand DNA sequences placed onto the scaffolds, with a “hinge” on the end of the double helices to hold a payload, like a cancer drug. They can hold multiple payloads at the same time and at desired positions on the origami structure, according to Zeng (Figure 4).
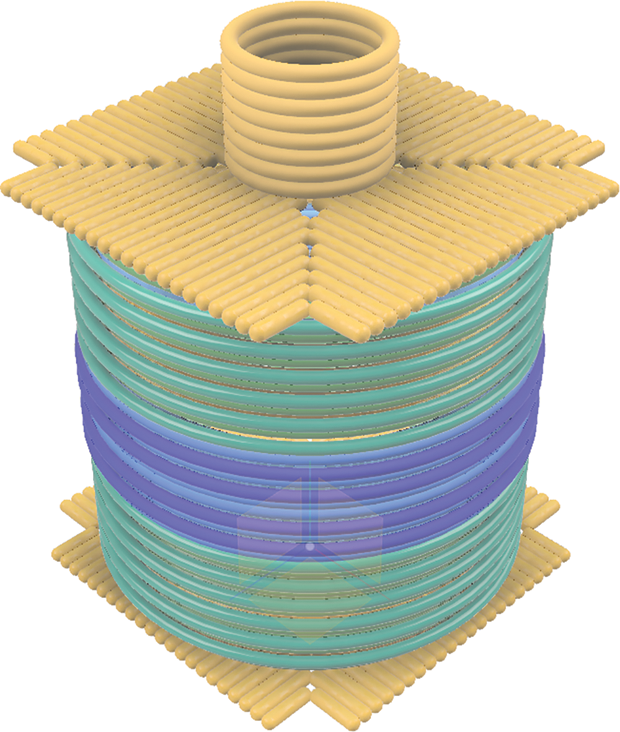
Currently, “we are developing a cancer vaccine using DNA origami,” she says. “The way the payloads—for example, tumor antigen and adjuvant—are arranged on the origami can create molecular signals for their receptor targets. When the device reaches the antigen-presenting cells, it enters and releases the payloads to the cells [to ultimately] generate a robust anticancer immune response.”
This technique lets one customize shapes and functions to load different types of things onto the DNA nanorobots, such as antigen and adjuvant or other ligands for cells to boost a patient’s immune response, or a tracking or imaging compound to more efficiently detect tumors.
While this organic nanobot approach is promising for nanomedicine, it faces similar limitations of other nanomedicine techniques, particularly cost, control, and figuring out how to translate it effectively for cancer immunology.
“Generally, the experts doing DNA origami research are in the synthetic biology area rather than cell biology and medical [areas]. Others in nanotech are coming from the engineering side. And clinicians typically don’t know a lot about these technologies,” says Zeng. “I’m hoping to bridge that gap by studying both the technology and clinical needs.”
One consideration for clinical applications is the possibility of unintended toxic side effects. An advantage to the organic approach is that the toxicity of this material is very low, according to Zeng. “Once you deliver the organic nanobot into the body, after it carries out its function, it could be digested by your cells like any other cellular debris,” she says.
Others are also accelerating research into nanorobotics for medicine, and closing in on clinical trials. Debabrata (Dev) Mukhopadhyay, PhD, director of the Mayo Clinic’s nanotechnology lab based in Florida, is close to finalizing phase-1 clinical trials for a nanotechnology-based drug delivery system targeting aggressive tumors.
“In addition to drug delivery, we aim to use nanoparticles to monitor tumor therapeutic outcome in real time,” says Mukhopadhyay, who points out that often when people are given chemotherapy, months go by before it’s clear whether the therapeutic drug is having an effect.
The lab also focuses on developing, testing, and applying nanorobotics to other medical areas, especially cardiovascular disease (delivering nanogel to repair arteries) and early-stage dementia (such as a biomarker nanoimaging system), as well as other immune disorders.
Mukhopadhyay, who draws his inspiration from the decades of interest in using materials like silver and gold for antimicrobial effects, cautions that any nanomedicine will need to be thoroughly tested for an immune reaction. Some of his work focuses on nanodiamonds, one of the lesser immunoreactive nanoparticles, which other researchers (such as Stanford professor Steven Chu) are investigating for a range of uses, including biomedical imaging.
Research into using nanorobotics and materials for cancer has been picking up speed in the last few years, and work also done at the Mayo Clinic has made progress in targeting specific cancer. Research led by Prof. Betty Kim showed a proof- of-concept that nanomaterials coated with antibodies to target a type of breast cancer receptor combined with molecules that activate the immune system, acting like flags on the cancer to help immune cells spot which tumorous cells to attack [4]. And in an advance in identifying cancer early, Prof. Shan Wang at the Stanford Center for Cancer Nanotechnology Excellence detailed an approach to use sensor technology and clusters of magnetic nanoparticles to attach to DNA and flag cancerous cells [5].
Despite these successes, it will be a while before we see the fully realized nanorobotics in use.
“Efficacy really needs to be proved in nanomedicine: Even with a passive dispersal of nanoparticles, there needs to be clear evidence that it’s more efficient than the existing methods. For the most part, the field as a whole is still in a proof-of-concept stage rather than a clinical-trial stage,” says Fischer. “There are encouraging scenarios where one can show that aspects work and maybe the dream of targeted, autonomous delivery systems can be realized, but it’s certainly a decade away at least.”
References
- Z. Wu et al., “A swarm of slippery micropropellers penetrates the vitreous body of the eye,” Sci. Adv., vol. 4, no. 11, Nov. 2018, Art. no. eaat4388. Doi: 10.1126/sciadv.aat4388.
- O. Felfoul et al., “Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions,” Nature Nanotechnol., vol. 11, no. 11, pp. 941–947, Nov. 2016. Doi: 10.1038/ nnano.2016.137.
- S. Betal et al., “Core-shell magnetoelectric nanorobot—A remotely controlled probe for targeted cell manipulation,” Sci. Rep., vol. 8, 2018, Art. no. 1755.
- H. Yuan et al., “Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy,” Nature Nanotechnol., vol. 12, no. 8, pp. 763–769, Aug. 2017. Doi: 10.1038/nnano.2017.69.
- S. X. Wang and C. Ooi, “Marrying nanomagnetics with RNA sequencing of single cancer cells,” in Proc. IEEE Int. Magn. Conf. (INTERMAG), Apr. 2018.