Water on Earth—in our oceans, rivers, lakes, and wetlands—might seem plentiful, but water that is clean and safe enough to drink actually isn’t so abundant. Nearly one in ten people still lacks access to safe water worldwide, according to the World Health Organization. In some of the world’s most remote and impoverished communities, people live with no taps, showers, flushing toilets, or nearby springs, making it difficult to keep water supplies safe from bacteria, chemicals, and particulates. Moreover, access to clean drinking water isn’t just a problem in the developing world; groundwater in developed countries is typically used far more quickly than it is being replenished. As the world population rises, growing numbers of thirsty people could exacerbate already-scant resources.

“So many people are suffering from lack of access to clean water, which means we need cost-effective, lower-power systems today that can help reduce the negative problems that arise from the global water crisis,” says Jeremy F. Lacey, chief executive officer (CEO) of Phoenix Revolution, Inc., a clean water company based in Boston, Massachusetts (Figure 1, right).
In response to these challenges, scientists, researchers, and companies are working on ways of delivering clean and safe water to more people—ranging from large-scale and sophisticated reverse- osmosis machines that can produce great quantities of water in places where water supplies are low but the ocean is nearby (see “Reverse Osmosis for Fresh Water”) to lower-tech options that allow individual households to eliminate from their water bacteria related to diseases such as cholera, dysentery, typhoid, and polio. Technologies using easily accessible, low-cost, natural materials have been the focus for many companies seeking to solve the world’s water problem.
[accordion title=”Reverse Osmosis for Fresh Water”]
While salt water constitutes the largest reservoir on Earth, water straight from the oceans cannot be consumed for daily use. However, many research teams worldwide have developed methods to enable the conversion of salt water to fresh water.
Many of those technologies hinge on the process of reverse osmosis (RO). Whereas osmosis is the natural flow of water from an area with many water molecules to an area with fewer water molecules, RO aims to move water in the opposite direction and against the concentration gradient. This process is made possible using a membrane to separate two compartments of water (one side for water that needs to be purified, and another side that houses the filtered water) along with energy to push the contaminated water against the membrane. Once a force is applied, the dissolved and suspended particles—including bacteria, viruses, salt, and organic materials—pile up on the pressurized side of the membrane, and pure water ends up on the opposite side.
Many companies have commercialized RO systems that can purify water for entire towns and communities. Not only can RO desalinate salt water, but these systems can also help clean up polluted rivers and lakes and remove pesticides from water in agriculturally intensive regions. For example, Phoenix Revolution, Inc., a company based in Boston, Massachusetts, has RO systems set up in ponds and lakes in New England, in addition to locations in India and China—all with the goal of providing safe drinking water to local communities (pictured below). “We’re interested in adapting our technology to any existing infrastructure,” says Jeremy F. Lacey, chief executive officer of Phoenix Revolution.

The thin-layered membranes that are critical components of RO systems look like ordinary sheets of paper—typically made from a cellulose derivative or polyamide—and have very small pores that allow only water through. While a cellulose acetate membrane allows water through at a faster rate, it can be ruined if it is not properly cleaned and maintained, because bacteria can accumulate and cause biofilms to form on the surface. Polyamide membranes, on the other hand, are more sensitive to chlorine—not to be confused with sodium chloride in saltwater, which is more stable—than cellulose membranes are. To work effectively, and to increase the life span of these filters, the membranes are typically protected by an additional layer. RO membranes used in brackish waters have an outer shell made of fiberglass, while membranes used for seawater have a tape wrap. Researchers are still optimizing ways to improve these membranes.
Because chlorine is known to damage RO membranes over time, some researchers, such as Sandra E. Kentish’s group at the Melbourne School of Engineering in Australia, are developing ways to bypass this limitation. “You can’t keep a standard desalination membrane clean by adding chlorine in the same way you keep your swimming pool clean,” Kentish says. Membranes damaged by chlorine are prone to bacteria buildup and the formation of biofilms, which would ultimately compromise the efficiency of the RO system. Using a layer-by-layer polymer assembly method, Kentish and her colleagues generated membranes with polysulfonate ions, which makes them more resistant to chlorine. While these membranes have been created and tested only at the bench, they can desalinate approximately 500 L of water in an hour—the equivalent amount of clean water a person would use in five days—if scaled up. Within desalination facilities, these membranes can convert 10,000 tons of salt water to fresh water in an hour.
Other researchers are integrating artificial water channels to desalination membranes. This idea is derived from the fact that, in our human cells, proteins known as aquaporins sit at the membranes and selectively allow water into the interior of cells while excluding other ions. Most membranes for RO are highly selective, but they are not very permeable. Chun-Long Chen, an engineer at the Pacific Northwest National Laboratory in Richland, Washington, thinks that integrating artificial water channels into membranes can help engineers generate membranes that are both very selective and permeable. Although much of this research is still being done at the bench, it could have a great impact when it’s ready for use in purification or desalination plants.
Nanoparticles offer yet another solution to improving membranes by integrating metals that can control the growth of biofilms or improve salt rejection. Some research teams have integrated silver, a natural disinfectant, into RO membranes to prevent biofouling. Tin oxide could also improve salt exclusion in RO membranes.
While RO has the potential to convert large amounts of water to safe drinking water, there are some limitations to the method. For one, trained personnel are needed to operate and maintain these systems to ensure a high quality of water output. Membranes can only last for so long, and if they are used beyond their life span, the quality of the purified water can be heavily compromised. And when membranes need to be replaced, costs can vary, depending on what types of membranes are used. Moreover, some RO units require copious amounts of energy to be operational.
[/accordion]
A Silver Lining

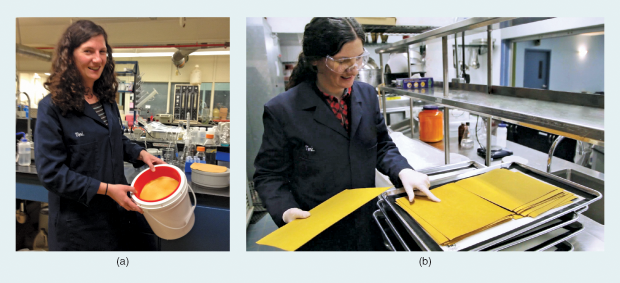
Folia Water, a company based in Philadelphia, Pennsylvania, has created a breakthrough water purification product utilizing the properties of silver (Figure 2) [1]. “It’s a well-known fact that silver is a disinfectant,” says Folia founder Theresa Dankovich (Figure 3). Folia uses silver-infused nanoparticles in its filters to eliminate viruses and other pathogens in water at a cost of less than a penny a day.
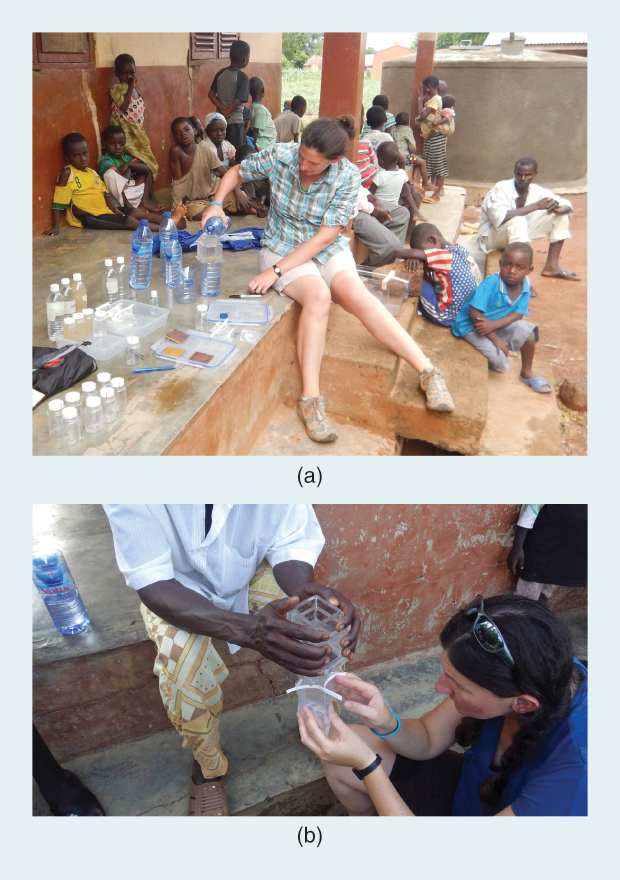
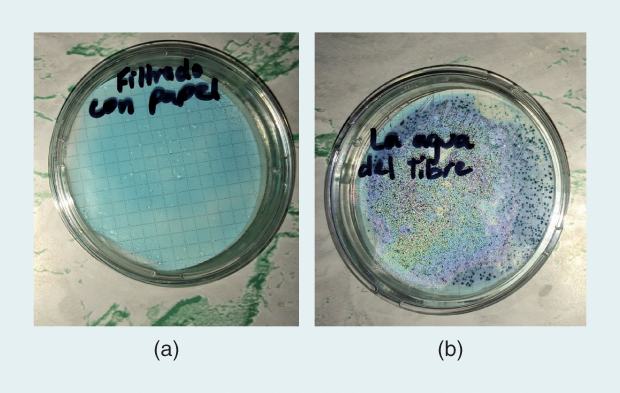
Although the company was formed only a year ago, the water filters are designed based on more than a decade of Dankovich’s research. Folia’s paper filters can be neatly folded into a cone to filter up to 100 L of clean drinking water; households, nonprofit partners, church organizations, and corporations in Ghana, Haiti, Kenya, and Bangladesh have bought and tested these filters in field trials (Figure 4). Then, the water purified during these field trials was analyzed by independent labs for microbial content, turbidity, and silver concentration (Figure 5). “We have gathered lots of useful user feedback and have already improved our product design based on this,” says Dankovich.
Watersprint, a company founded in 2013 in Lund, Sweden, has created devices powered by solar panels that employ ultraviolet light-emitting diode (UV-LED) technology to filter water. The use of solar panels precludes the need for electricity, which is suitable for communities that lack infrastructure for power. Solar energy fuels the UV-LED lights, which, in turn, damage the DNA in living organisms, such as bacteria and viruses.
“These units basically require no maintenance,” says Linda Kokkola, CEO of Watersprint. They can be used in camper vans and recreation vehicles, and Watersprint has sold about 250 units in the last year and a half; more importantly, the company has been able to bring this technology to areas of Bangladesh that lack clean water. A single unit can cost up to 11,000 Swedish kronor (approximately US$1,228). Although the device can disinfect bacteria and viruses in water—thereby lowering the incidence of diarrhea, Legionnaire’s disease, and other bacteria-borne water diseases—it might not be as effective in ridding water of heavy metals and other organic pollutants. “UV treatment alone is not sufficient to remove metals,” says Kenneth M. Persson, one of Watersprint’s founders. “It must be followed by filtration, adsorption, or ion exchange. I think we will be able to offer solutions for this in the next year.”
Another Swedish company, Vestergaard, with main offices in Lausanne, Switzerland, manufactures Lifestraw, a device that can remove nearly all waterborne bacteria through a superfine filter without power and can filter up to 1,000 L of water. The straw is incompatible with salt water, but its hollow fiber membrane traps microorganisms, including waterborne pathogens that cause cholera, dysentery, giardia, hepatitis E, and typhoid. The straw is being used in Latin America and some countries in Africa.
The Miracle of Moringa Trees

Adrian Rennie, a physicist at Uppsala University in Sweden, has investigated how moringa seeds could be used to filter water (Figure 6, right) [2]. Ancient Egyptians are thought to have crushed seeds from moringa trees to clear up cloudy water. The protein component of the moringa seeds causes impurities in water to cluster together, and those aggregates can be removed in a separate step, ultimately filtering out bacteria, dyes, and heavy metals from contaminated water (Figure 7). Because moringa trees grow readily in areas of the world that are commonly water stressed and lack access to clean water, such as Africa, southern Asia, and South America, this approach is perhaps one of the most accessible ways for communities to filter water. And moringa seeds are readily scalable: depending on how much water needs to be filtered, the ground seeds can help a villager filter a few liters of water at a time, or it can help provide entire towns with a clean water supply. Already, some water treatment plants in Malawi, India, and Brazil are using moringa seeds to filter water on a larger scale.

Similarly, Suresh Valiyaveettil, a chemist at the National University of Singapore, has come up with another low-cost idea to filter water that could be used in resource-poor settings: fruit peels [3]. Depending on how much water needs to be purified and the contamination level of the original water source, more peels can be added to treat the water. Fruit peels contain cellulose, a sugar that makes up most of a plant’s cell walls. Cellulose has chemical groups that can attract metals and, in this application, filter out metallic impurities in water. “It’s very easy to scale up as long as you have access to sufficient fruit peels, which is not a big problem in many places,” says Valiyaveettil. Although this method is still in its early research phases, it holds great potential for use in the field, particularly in low-resources settings. Currently, these fruit peels have been able to remove heavy metals and dyes from water; it has yet to be shown whether these peels can also filter out bacteria and viruses.
Cleaning Up Fracking Water

Researchers are also looking into ways to treat fracking water, liquids that are laden with a number of toxic heavy metals and organic and inorganic contaminants. In 2013, a research team led by Zhi-Gang Feng, an engineer at the University of Texas, San Antonio, investigated ways to treat flowback water—the fluid that returns to the surface after fracking, which is laden with chemical additives and dissolved metal ions—using biochar derived from wood chips of pine and oak trees to create an economical and environmentally friendly solution (Figure 8). While this method has yet to be employed in the field, lab tests indicate that biochar can remove more than 85% of the flowback water so that it is safe for reuse. And, while this method was initially developed for fracking water, Feng and collaborator Maoqi Feng at the Southwest Research Institute in San Antonio believe that, in the future, it can be combined with other methods to purify water heavily laden with contaminants (Figure 9).
Although this project was born to address the water affected by fracking in the United States, other countries in the world—Russia, China, Argentina, Poland, Turkey, the Ukraine, and Libya [4]—have abundant amounts of shale that could potentially be exploited by fracking. A single well of flowback water generated from fracking could contain anywhere from 1 to 5 million gallons of toxic fracking fluids.

In the United States, chemicals from industrial plants or military bases can leach into water. A study from Harvard University in Cambridge, Massachusetts, estimates that more than 16 million Americans are drinking water contaminated with perfluorinated alkyl substances, chemicals that are thought to be linked to hormone fluctuations, immune system disorders, high cholesterol, and obesity [5]. Evoqua Water Technologies, a water treatment company headquartered in Warrendale, Pennsylvania, has developed a technique to remove these chemical contaminants. Evoqua uses granular activated carbon to filter toxic chemicals. “Activated carbon is considered the best-available technology for the removal of PFCs [perfluorochemicals] from drinking water, and the use of coconut-shell to produce activated carbon is an excellent use of a product that would otherwise be considered waste,” says John Lombardo, Evoqua’s product manager. Not only does the activated carbon remove contaminants from a stream of water; the pores of these activated carbon materials can also sequester chemicals to the carbon bed.

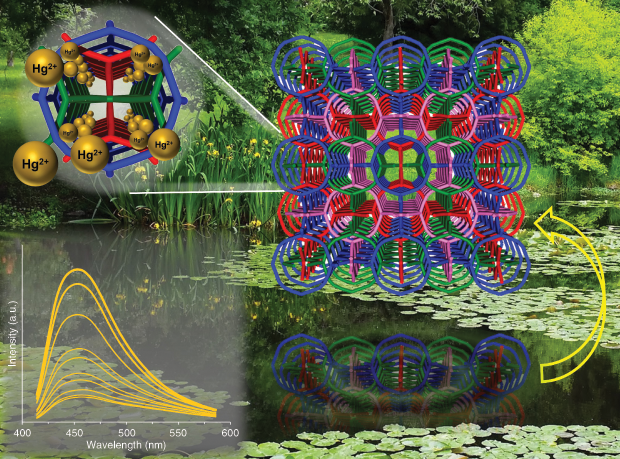
More recently, researchers from Rutgers University in New Brunswick, New Jersey, and the Lawrence Berkeley National Laboratory in Berkeley, California, have been motivated to develop new ways to remove heavy metals from drinking water after cases in Flint, Michigan, and Newark, New Jersey, were publicized. Jing Li (Figure 10, right) and her colleagues at Rutgers have developed luminescent metal-organic frameworks (LMOFs)—lattices made of organic material—to capture heavy metals, such as mercury and lead [6] (Figure 11). While LMOFs are typically used as sensors for agricultural toxins or in bioimaging, these chemicals are ideal for ridding water of heavy metals. “They’re the perfect material candidate for sensing and capturing heavy metals,” Li says. “They’re porous and easy to modify, so we can target a specific contaminant.” Much of this technology is still being developed at bench scale, but Li hopes that LMOFs can eventually be incorporated into membranes for water purification to capture metals on a broader scale.
Despite the challenges of attaining clean water in both low-resource settings and more developed countries, the technologies and advances discussed here demonstrate that scientists and companies recognize the imperative need to utilize many different methods to obtain clean, safe water. Whether it is using silver filters, organic lattices, or fruit peels that can be taken out of the compost bin, a range of tools exists to obtain safe water.
References
- T. A. Dankovich and D. G. Gray, “Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment,” Environ. Sci. Technol., vol. 45, no. 5, pp. 1992–1998, Feb. 2011.
- M. S. Hellsing, H. M. Kwaambwa, F. M. Nermark, B. B. M. Nkoane, A. J. Jackson, M. J. Wasbrough, I. Berts, L. Porcar, and A. R. Rennie, “Structure of flocs of latex particles formed by addition of protein from moringa seeds,” Colloids Surf., vol. 460, no. 20, pp. 460–467, Oct. 2014.
- M. Ramakrishna, L. Xuanjun, A. Adin, and S. Valiyaveettil, “Fruit peels as efficient renewable adsorbents for removal of dissolved heavy metals and dyes from water,” ACS Sustainable Chem. Eng., vol. 3, pp. 1117–1124, Apr. 2015.
- J. McBride and M. A. Sergie. (2015, June). Hydraulic facturing (fracking). Council on Foreign Relations. [Online].
- A. Watkins, C. Wood, M. Lin, and B. Abbott. (2013, June). Effects of perfluorinated chemicals on adipocyte development. [Online].
- N. D. Rudd, H. Wang, E. M. A. Fuentes-Fernandez, S. J. Teat, F. Chen, G. Hall, Y. J. Chabal, and J. Li, “Highly efficient luminescent metal-organic framework for the simultaneous detection and removal of heavy metals from water,” ACS Appl. Mater. Interfaces, vol. 8, no. 44, pp. 30,294–30,303, 2016.



