Nearly two decades ago, Hossam Haick was working on a Ph.D. degree in chemical engineering at the Technion Israel Institute of Technology when terrible news hit: his friend and officemate had leukemia. It was Haick’s first close encounter with cancer and the physical toll its treatment could wreak on a person. “He’s fine now, but it was very difficult to see him suffer,” Haick remembers.
At the time, the Israeli scientist had no experience at all with medicine. He’d grown up on a diet of physics texts from his father’s library, and at Technion he was working on photocatalysis, ways that light energy could degrade, say, pollutants. Still, the experience planted a seed in the engineer’s mind. Over the next few years, Haick progressed through postdocs at the Weizmann Institute of Science in Tel Aviv, then at CalTech in the United States, developing an expertise in nanotechnology and chemical sensors for electronic devices. But, on the side, he read literature on cancer—how it was treated and, most important, how it was detected. “I saw more and more how early diagnosis of the cancer might increase the survival rate, even with just currently available treatments,” he says.
Then in 2006, as Haick’s CalTech postdoc was winding down and the young engineer was struggling to envision what a lab of his own might focus on, a flurry of news articles appeared reporting on dogs trained to detect cancer in the breath and skin of patients. A light bulb went off in Haick’s head: “I thought, why can’t we learn from the dogs, imitate the canine olfactory system, and use it for the early detection of disease?”
And so, despite a lack of formal training in medical research or even a clear grasp on how medical dogs performed their feats, he accepted an assistant professorship from his alma mater, Technicon, and told them that he would be working on cancer. Specifically, he would develop a cheap, noninvasive tool that would sniff out traces of early lung cancer based on people’s breath (Figure 1).
Dogged Research
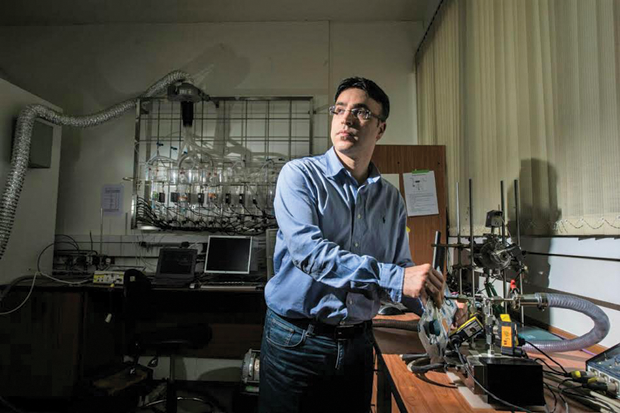
After he accepted the position, Haick won a US$2.2 million Marie Curie Excellence Award from the European Commission for promising new researchers to help them get started. Today, Haick is a full professor at Technion, overseeing nine labs, three international consortiums, and two startups based around what he calls Na-Nose—as in a nanotechnological e-nose.
Remarkably, his first functional proof of concept actually came within the first two years of the project. As it turned out, Haick learned early on that science had long known certain key chemicals tended to be reliably elevated in the breath of people with certain cancers, particularly lung cancer, compared to healthier people. These included hydrocarbons like ethane and various aldehydes, for instance, which are produced by cell membranes as a result of oxidative stress. There was no single biomarker; rather, it was the pattern of common chemicals that was key, like a fingerprint. The main obstacle to applying this knowledge was the equipment—available technologies like mass spectrometry were expensive, time-consuming, and difficult to use without expertise.
In contrast, Haick’s prototype was easier to use and had the potential to be much cheaper than typical methods. The carbon nanotube-based prototype device was painted with organic films that would capture and respond to different chemicals released in breath vapor by changing shape—thus changing the electrical resistance of the nanotubes and the digital out- put. In the lab, the device was able to separate almost perfectly the vapor fingerprints of healthy people from those with lung cancer (Figure 2).

Even so, the technology’s first trials in the clinic didn’t go very well—accuracy dropped to near chance. But over the following decade, Haick and his team tweaked and refined the concept, experimenting with different nanoshapes and materials and coating them with more specific chemical receptor films and waterproofing layers. Accuracy in the clinic crept back up to 76%, then 88%. The team learned to tailor the device to new diseases, including kidney disease, gastric cancer, and multiple sclerosis.
Much of this research was undertaken with the traditional scientist’s air of curiosity and gut feeling. But, Haick admits, there have been a few unexpected occasions when he took on an idea more to rule it out than anything else. That was the case with Parkinson’s disease. He’d had an outlier in his data from a patient who just happened to have that disease. And so, sure that it was just a fluke, he set up a quick pilot. “I even guaranteed it would not succeed,” he says. “I thought, it’s in the brain, why would it come to the lungs?”
But when multiple blind studies he conducted discriminated healthy patients from those with Parkinson’s (as did replications in Scotland, China, and Germany), he was convinced. “There is sometimes a balance between curiosity and skepticism,” he says. He’s now also running animal studies to try to find out how that chemical signature happens.
Over the years, Haick has steadily accumulated recognition for his research. In 2008, Technology Review included him on its list of the world’s top 35 young scientists; two years later, he earned a Knight of the Order of Academic Palms. Late last year, Haick’s efforts finally culminated in a massive project in which he and an international team of collaborators were able to use the latest version of his Na-Nose tool (Figure 3) to diagnostically distinguish 17 disease breathprints from the breath of 1,404 people in nine clinical centers around the world with an average accuracy of 86%.
Simple Challenges

For Haick, it’s the challenge of the research and invention that drives him forward. But with his basic Na-Nose project, that’s begun to lag. Much of what remains is simply expanding and refining it: more diseases, more clinical tests. “This is good—but in terms of the science, per se, for me, it’s a little bit boring,” he admits. So more and more, Haick has begun to hand the reins over to companies and clinical researchers to run the studies themselves while he explores new frontiers.
The extent of Haick’s work now spans not just diagnostics but also food safety and biometric security applications. Five companies have licensed the technology, and two spinoffs have emerged from his lab. One such is NanoVation-GS, started in 2014 and built around the idea of a nanotech skin patch for hospitals designed to go under the nose and capture the vital signs like blood oxygen saturation, temperature, pulse, and breathing rate currently monitored by multiple machines. A similar project is underway with the help of the Bill and Melinda Gates Foundation to create a flexible, self-healing polymer skin patch to detect tuberculosis for use in developing countries. Data aren’t published, but Haick says that so far the team has proven both that tuberculosis does have reliable chemical signatures and that, in a clinical trial with roughly 600 volunteers in India, these signatures actually can be detected by early prototypes of the skin patch technology.
The concept is the same as his Na-Nose breathalyzers—skin emits volatile compounds just as the lungs do. The procedure still involves nanotech electrical conduction modulated by patterns of activity from organic, chemical-sensing films: however, the requirements are different. The tuberculosis patch, in particular, has to be extremely user friendly and self-powered, hold up to the daily grind of life in a developing country, and, most important, be extremely inexpensive. “In my lab, I’m used to developing very complicated systems under vacuum conditions,” Haick says. “To go to this sort of simplicity of use—it is a huge challenge.”
Now, over a decade into his career, the engineer has become an old hand at medical research, having been immersed in clinical studies and even in vitro cell cultures and animal research. But he credits much of his success to his initial unfamiliarity with the field of medicine and biology when he was first starting out.
“It was one of the more brilliant things that happened to me—that I didn’t understand what is medicine,” Haick says. “I could allow myself to imagine everything, even things that looked unrealistic to a medical doctor.”
“Sometimes, ignorance is a good thing to happen in science.”