Contact lenses offer an ideal position to monitor glucose and other health factors, but developing safe and easy-to-wear smart lenses remains a challenge
Contact lenses are ideal conduits for continuous health monitoring. They have a long safety record, and they sit on the eye, where they have access to a range of biological signals. Making the transition from vision correction to biological monitoring, however, requires advances in technological development so the lenses not only detect and report signals accurately, but retain the high level of comfort that users have come to expect.
It has been nearly a decade since Google and pharmaceutical giant Novartis announced their intent to create a smart contact lens that could detect glucose levels in tear fluid as a way to help diabetic patients [1]. Engineer and current-day smart lens researcher Chi Hwan Lee (Figure 1) remembers that time well: “I was in post-doc training at the University of Illinois when I saw an article about it. I was very fascinated by that technology, but I knew it would face many challenges.” He was right. The Google/Novartis project and others have come and gone, but the quest continues with several research groups, including Lee’s, pursuing smart contact lenses for health-related purposes.

Figure 1. Chi Hwan Lee, Ph.D., associate professor of BME and mechanical engineering in the School of Mechanical Engineering at Purdue University, West Lafayette, IN, USA. (Photo courtesy of Chi Hwan Lee.)
Glucose levels remain a prime target for smart contact lenses. Another is internal fluid pressure in the eye (intraocular pressure or IOP), which is a major risk factor for glaucoma, noted smart lens developer Sei Kwang Hahn, Ph.D., professor in the Department of Materials Science and Engineering at Pohang University of Science and Technology (POSTECH) in Pohang, South Korea (Figure 2). “Blood glucose levels and IOP show significant variations over time, so continuous monitoring is very important, (and) smart contact lenses are especially promising for health care applications, because they can be used as an excellent interface between the human body and the electrical device.”

Figure 2. Sei Kwang Hahn, Ph.D., professor of materials science and engineering at POSTECH. (Photo courtesy of Sei Kwang Hahn.)
Printing biocompatible polymers
One of the major challenges in smart-lens efforts has been incorporating all of the needed electronic components into easy- and safe-to-wear contacts, according to Lee, Ph.D., who is now an associate professor of BME and mechanical engineering at Purdue University, West Lafayette, IN, USA.
“Initially, I tried using a silicon material for the integrated circuit (IC) chips, just like Google did, because that’s the well-established material for electronics. I thought that by simply miniaturizing those chips, we could minimize the discomfort level and the risk of wearing them because, after all, silicon is like putting a piece of glass on your eye. But no matter how I miniaturized it, clinical-test feedback from clinicians and patients was never favorable,” Lee said. He also had to develop the electronic components so they could stand up to everyday user handling as well as the highly reactive disinfecting process, which includes soaking the lenses in a hydrogen peroxide solution overnight.
Those difficulties led Lee down a new path: switching to polymer-based electronic materials and incorporating the technology onto already-on-the-market soft contact lenses. To do it, his group synthesized biocompatible, conducting, 3D-printable polymers [2]. He described, “We use a commercial, automatic 3D dispenser that can print our device pattern using different materials, such as the conducting material and insulation material, onto a temporary water-soluble film. And then we dissolve this water-soluble layer, leaving the device layer attached to a commercial contact lens.” This also required a “wet glue” to keep the device in place, and for that, Lee turned to Purdue chemical engineer and professor Bryan Boudouris, Ph.D., who took inspiration from marine mussels to produce a strong polydopamine adhesive.
The electronics integrate a capacitance sensor configured into an inductive antenna design, which monitors IOP by continuously measuring pressure-related changes in the curvature of the eye [3]. “The continuous monitoring of IOP is very valuable data because IOP fluctuates morning to afternoon to evening, season to season, even by your posture. And once your IOP goes beyond a certain value, the increased pressure can damage your optic nervous system,” Lee explained. Eye-drop medications are already available to decrease the dangerous pressure, so if patients have access to continuous, real-time IOP measurements, they can apply the eyedrops to mitigate pressure swings.
In appearance, the overall device looks like a narrow ring that sits toward the periphery of the lens (Figure 3), so it has no impact on vision, he said. Not only that, “the device material itself is also more than 10 times softer than the contact lens, more than 10 times more stretchable than the contact lens, and more than 10 times thinner than the contact lens, so it does not affect the wearability or other properties of the contact lens much.”
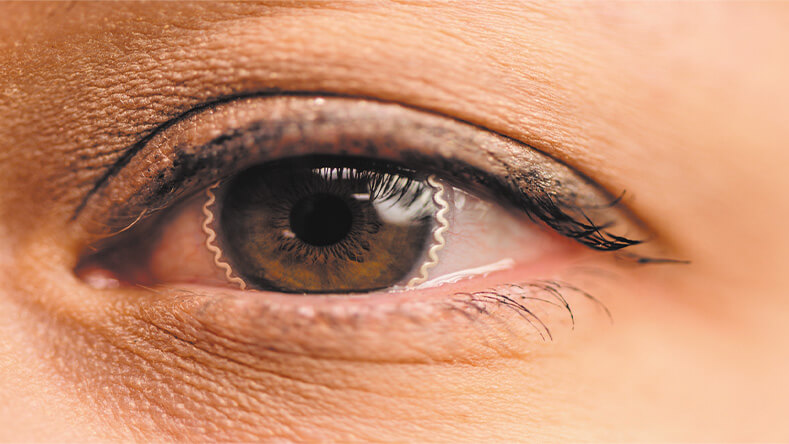
Figure 3. Lee and his group are adding electronics (the gold ring) to commercial soft contact lenses so they are able to continuously monitor internal fluid pressure in the eye (intraocular pressure or IOP), which is a major risk factor for glaucoma. Continuous monitoring is important because IOP varies over time and requires medication when it reaches a certain level. Lee is co-founder of a startup company, BVS Sight in Indianapolis, that is working on hardware and software development of the accompanying patient-alert system, as well as beginning steps toward regulatory approval and ultimate commercialization. (Photo courtesy of Purdue University/Rebecca McElhoe.)
Lee’s research group is now running the lenses through clinical testing and using patient feedback to optimize the design. Commercialization will come next, and Lee has already co-founded a startup company, BVS Sight in Indianapolis, to handle market analysis, risk assessment, both hardware and software development of the accompanying patient-alert system, and the regulatory pathway to obtain ultimate FDA approval.
As this effort proceeds, he and his research group at Purdue are moving forward with other projects, such as adapting the soft contact lens platform to monitor glucose levels from tear fluid and developing smart soft contact lenses that can detect and measure dry eye syndrome and infections. He remarked, “We keep exploring other possibilities and more expanded applications.”
Contacts in South Korea
One of the possibilities explored by Hahn and his POSTECH group is developing smart contact lenses that combine diagnostic and therapeutic capabilities (Figure 4). This includes their work on a lens that detects glucose levels in tears [4] and responds by administering medication to treat diabetic retinopathy, and another that detects IOP and delivers glaucoma medication [5].
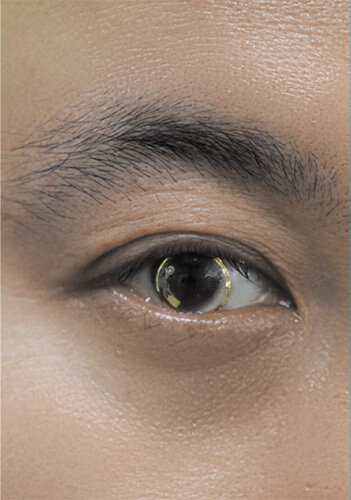
Figure 4. Hahn’s POSTECH research group is developing smart contact lenses to not only monitor IOP related to glaucoma, and glucose levels associated with diabetic retinopathy, but also to respond by delivering medication directly to the eye. Hahn co-founded the company PHI BIOMED further advance both projects and bring them through the regulatory pathway and to commercialization, hopefully in 3–5 years. (Photo courtesy of Sei Kwang Hahn.)
Working in collaboration with other researchers in the United States and South Korea, Hahn’s group constructed lenses using a silicon hydrogel, which allows the gas permeability needed for long-term wear, Hahn said. Currently, the smart lens is about 200 μm thick, which is about twice the thickness of a typical commercial lens, but “much thinner than the 580 μm [6] of the previously FDA-approved Triggerfish (smart lens for glaucoma) commercialized by SENSIMED company,” he noted. “We are currently trying to reduce the thickness of our smart contact lens to around 150 μm for patient compliance.”
The smart retinopathy-related contact lens contains ultrathin, flexible electrical circuits and a microcontroller chip for real-time electrochemical glucose biosensing; on-demand, controlled drug delivery; wireless power management; and data communication. “Glucose oxidase on the sensor binds with glucose, triggering an electrical current change, which is proportional to the amount of glucose,” Hahn explained. The lenses also include tiny drug reservoirs that are sealed with gold membranes. When an electrical current is applied, the membranes dissolve, triggering the release of the medication. The researchers are currently designing the system to deliver medications either automatically or via smartphone control by the patient.
“In the case of smart IOP contact lens, we first focused on the development of IOP sensors using gold hollow nanowires with high sensitivity, stability, and biocompatibility. Then, the structural design of the drug-delivery system was optimized for high loading and efficient release of therapeutic drugs,” Hahn said. With additional work to fully integrate the sensor, drug-delivery system, IC chip, and electrodes on the same substrate, they were able to design a lens to continuously monitor IOP and deliver the proper dose of drugs on demand and directly onto the eye.
So far, they have conducted animal testing of the glucose and IOP theranostic lenses with good results, and now hope to begin clinical testing and to start the regulatory approval process with a goal of commercializing the lenses in South Korea within three years, and in the United States within five years.
Beyond those projects, Hahn’s group is working on another smart retinopathy lens that incorporates light-emitting diodes (LEDs) and a photodetector [7]. When glucose binds to certain chemicals, it generates light, which can be picked up by the photodetector, he described. “Besides analyzing the amount of glucose in tears, the LEDs can also then supply light therapy, which may reduce retinopathy symptoms.”
Other potential applications for smart contact lenses exist as well, and Hahn is pursuing some of those through his new company PHI BIOMED and collaboration with the contact lens company INTEROJO of Pyeongtaek, South Korea. Future projects include theranostic lenses for age-related macular degeneration and other chronic diseases, and smart contact lenses for vision correction, including myopia, he said. “We are working on the commercialization of smart biosensing contact lenses, smart drug-delivery contact lenses, and smart theranostic contact lenses … one by one.”
References
- B. Otis and B. Parviz. (Jan. 16, 2014). Introducing our smart contact lens project. Alphabet Blog. Accessed: Apr. 12, 2023. [Online]. Available: https://blog.google/alphabet/introducing-our-smart-contact-lens/
- K. Kim, B. Kim, and C. H. Lee, “Printing flexible and hybrid electronics for human skin and eye-interfaced health monitoring systems,” Adv. Mater., vol. 32, no. 15, Apr. 2020, Art. no. 1902051.
- J. Zhang et al., “Smart soft contact lenses for continuous 24-hour monitoring of intraocular pressure in glaucoma care,” Nature Commun., vol. 13, no. 1, p. 5518, Sep. 2022, doi: 10.1038/s41467-022-33254-4.
- S. Kim et al., “Bimetallic nanocatalysts immobilized in nanoporous hydrogels for long-term robust continuous glucose monitoring of smart contact lens,” Adv. Mater., vol. 34, no. 18, May 2022, Art. no. 2110536, doi: 10.1002/adma.202110536.
- T. Y. Kim et al., “Wireless theranostic smart contact lens for monitoring and control of intraocular pressure in glaucoma,” Nature Commun., vol. 13, no. 1, Nov. 2022, Art. no. 6801, doi: 10.1038/s41467-022-34597-8.
- R. Hubanova et al., “Effect of overnight wear of the Triggerfish sensor on corneal thickness measured by Visante anterior segment optical coherence tomography,” Acta Ophthalmol., vol. 92, no. 2, pp. e119–e123, Mar. 2014, doi: 10.1111/aos.12241.
- G. Lee et al., “Smart wireless near-infrared light emitting contact lens for the treatment of diabetic retinopathy,” Adv. Sci., vol. 9, no. 9, Mar. 2022, Art. no. 2103254, doi: 10.1002/advs.202103254.



