Sleep disordered breathing in children ranges from snoring, which has a prevalence of 12%, to obstructive sleep apnea (OSA) syndrome, which has a prevalence of 2–3% in the general population [1]. The underlying causes of pediatric OSA are extremely complex. There are bony structural influences, as seen in craniofacial abnormalities, and soft tissue abnormalities, such as a large tongue, redundant soft tissue, or compliance/collapsibility issues. In some groups, such as those with Down syndrome, a combination of these factors comes into play.
Sleep-disordered breathing has significant adverse effects on the lives of children and their families. Interrupted sleep in children can lead to sleepiness during normal activities and learning difficulties and can aggravate attention deficit disorder and other behavior disorders. In normal children, removal of the tonsils and adenoids is highly effective in treating OSA and snoring, but there is a subset of patients who only partially respond to this first-line treatment. Children who often continue to have significant OSA after adenotonsillectomy include older children as well as those with asthma, obesity, metabolic syndrome, craniofacial abnormalities, and Down syndrome [2]. When persistence of OSA is documented by polysomnography, further treatment is often needed. The first line of treatment is continuous positive airway pressure applied by a face mask or nasal mask during sleep. However, patient compliance is a significant problem, especially in children with a limited capacity to understand the need for wearing the apparatus.
This difficultly often brings patients back to their medical provider to find other options for treatment. For moderate or severe persistent OSA in children, this often bring us to surgical options including repeat adenoidectomy, lingual tonsillectomy, midline posterior glossectomy, tongue suspension, supraglottoplasty, pharyngoplasty, or nasal turbinate reduction and septoplasty. However, the effectiveness of surgical treatment of pediatric patients with refractory OSA has only been about 60% [3]. Most of the surgical techniques have focused on making the airway larger or suspending tissue so that it is less liable to collapse. These are largely anatomical methods of addressing the issue, and they ignore many of the neurophysiological and tissue compliance factors that affect the collapsibility and obstruction of the airway. We believe there is a real opportunity here for novel imaging tests, based on magnetic resonance, to enable a breakthrough in our understanding of pediatric OSA and the ability to select optimal therapy.
Imaging Methods
Static anatomical imaging of the upper airway is easily performed on current commercially available magnetic resonance imaging (MRI) scanners. Established protocols include three-dimensional (3-D) axial and sagittal T1- and T2-weighted fast spin-echo scans, each taking roughly 2–4 minutes during free breathing [4], [5]. Patient motion is typically not a problem during wakefulness. However, during natural or drug-induced sleep (sedation or general anesthesia designed to simulate sleep), patients often thrust their tongue and jaw. In such cases, it is helpful to use respiratory triggering during expiration, when the airway is largest and most static. These static images can identify the recurrent adenoid tonsils, enlarged lingual tonsils, elongated soft palates, enlarged tongues (relative to the oral cavity), and areas of narrowing, all of which are amenable to surgical intervention.
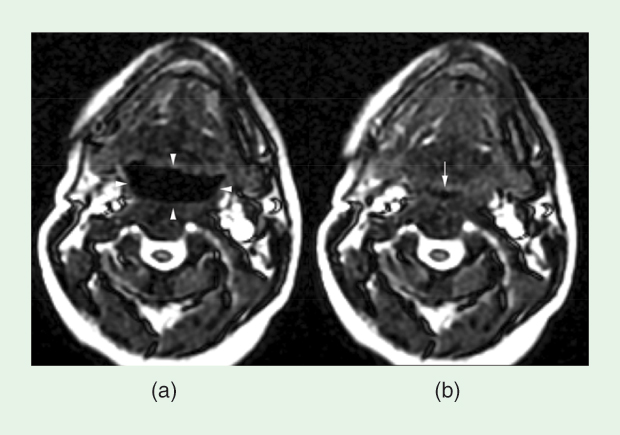
Dynamic two-dimensional (2-D) imaging of the airway is also easily performed on current MRI scanners. Typical temporal resolution of the images is from 250 ms to 500 ms using a 2-D gradient echo or balanced steady-state-free-precession real-time imaging sequence. Images are obtained over a 30-second (or longer) acquisition in the midline sagittal plane, axial in the retroglossal airway just above the epiglottis, and oblique plane (transaxial to the airway) through the nasopharyngeal airway. Displaying the images as a movie provides a picture of the airway dynamics. Numerous studies have shown the airway to be more dynamic in patients with OSA resulting in ballooning during expiration and collapse during inspiration, as shown in Figure 1 and Movie 1 (to view all movies mentioned in this article, see the supplemental material that accompanies this article in IEEE Xplore). It is the combination of anatomy and dynamics that aids the surgeon in clinical practice to better target the therapy. For instance, cine MRI of patients while they are awake or under drug-induced or natural sleep has been shown to help inform surgical decisions and improve outcomes [6].
A number of new methods are emerging for the study of airway dynamics that, for now, require specialized MRI software or an experimental apparatus. These techniques incorporate a combination of parallel imaging, compressed sensing, novel gating strategies, and novel stimuli. Compressed sensing (or constrained reconstruction) strategies have recently been shown to work particularly well for cine 2-D and 3-D proton density weighted airway imaging because the resulting movies are sparse in total variation (or generalized total variation) and have small regions that are moving compared to the full slice or volume being imaged. Novel gating strategies and stimuli allow for synchronization with respiration on conventional hardware [7] and the study of airway tissue compliance during wakefulness and sleep [8].
One nascent method is real-time 3-D imaging of the upper airway using compressed sensing [9]. The latest technology is able to achieve 1.8–2.0-mm spatial resolution, with 600-ms temporal resolution, which enables visualization of the entire airway during naturally occurring apneas [10], as shown in Figure 2 and Movie 4. A current limitation of this technology is acoustic noise, and it remains to be seen if patients can reach rapid eye movement sleep during this type of scan.
![FIGURE 2: Real-time 3-D imaging enables complete visualization of events during natural sleep. Shown here are data from a 13-year-old male primary snorer. The waveforms shown are (a) mask pressure, (b) abdominal respiratory effort, (c) heart rate, and (d) oxygen saturation simultaneously measured during an MRI scan. Representative axial and sagittal slices are shown for two time frames (e) prior to (red dots), and (e) during (blue dots) an obstructive apnea. A 25-second obstructive apnea is indicated by zero mask pressure, and respiratory effort (black arrows) total airway collapse is observed in the retropalatal slices (yellow arrows compared to white arrows). The oxygen saturation drop is observed after the obstructive apnea [magenta arrow in (d)]. (Please also see Movie 4 in IEEE Xplore.)](https://www.embs.org/wp-content/uploads/2014/09/nayak02-2339398.jpg)
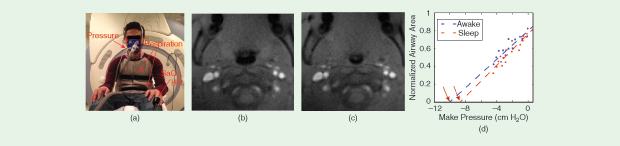
Another nascent method is active airway compliance testing, which involves the generation of negative pressure via inspiratory load (one to three breaths) [8] during ultrafast 2-D imaging of the airway in cross section [11]. Under these conditions, airway motion is extremely rapid requiring at least 10 frames/s and millimeter spatial resolution. As shown in Figure 3 and Movie 5, this enables in-vivo measurement of the pressure–airway area relationship. Extrapolation of these measurements provides insight into the airway closing pressure, called Pcrit. Such experiments can be repeated to reveal differences in the closing pressure between, for instance, wakefulness and sleep. These scans could also provide information about regional soft-tissue mechanical properties, and exactly how to do this is an active area of investigation.
Cine MRI is advantageous for studying the anatomy and neurophysiology of the upper airway because it allows for the observation, recording, and repetition of experiments in an individual under a controlled situation without radiation or endoscopy. A real-time or retrospective analysis of airflow, anatomy, and dynamics of the entire upper airway can yield detailed information about neurophysiological response and tissue compliance. The airway can be observed and recorded during wakefulness, natural sleep, or drug-induced “sleep” before and after changing variables to observe neurophysiological changes, which are often different in patients with OSA, resulting in an airway that is more susceptible to collapse. Imaging can inform patient-specific computational models that can be used to test the effects of surgery and determine the best surgical approach or develop new approaches [12].
Conclusion and Perspective
MRI is an emerging clinical method for evaluating the airway in OSA and can be accomplished on all standard commercial MRI units. MRI is used at only a few centers to guide treatment decisions, but this number is growing as more otolaryngologists train at centers that use this modality routinely. Growth, however, depends on having imagers and surgeons collaborate to build a program. MRI at many of these centers is being used to investigate the anatomical and neurophysiological factors that contribute to OSA.
Centers performing research investigations are a glimpse of the future. Advances in technology, including silent imaging modes [13], four-dimensional imaging of airway dynamics using compressed sensing [9] and layering with sleep-lab technology, could further enhance the role of imaging in the treatment decision process for patients with refractory OSA. Currently, these are works in progress at various centers across the country and are being used to discover generalizable information to help guide treatment in the future. Ultimately, one can imagine having an MRI sleep lab to inform the decision making in treatment of refractory OSA with physiologic phenotyping of patients that allows for optimal treatment and better outcomes. Additionally, such an MRI sleep lab could be used to study the effects of nonsurgical treatment on the airway, such as neuromuscular stimulation or drugs.
The best medicine is the prevention of disease, but, once a disease develops, a cure is the next goal. In refractory sleep disordered breathing, a cure has been multifaceted and elusively difficult. This is perhaps due to an incomplete understanding of the physiological and anatomical underpinnings of OSA mechanisms. MRI, in combination with sleep techniques, is one promising tool that can provide deeper insight for better treatments.
Acknowledgment
We acknowledge grant support from the National Institutes of Health (R01-HL105201 and R01-HL105206) and thank our many collaborators. In particular, we thank Yoon-Chul Kim and Ziyue Wu for contributing figures.
References
- J. C. Lumeng and R. D. Chervin, “Epidemiology of pediatric obstructive sleep apnea,” Proc. Am. Thorac. Soc., vol. 5, no. 2, pp. 242–252, Feb. 2008.
- R. Bhattacharjee, L. Kheirandish-Gozal, K. Spruyt, R. B. Mitchell, J. Promchiarak, N. Simakajornboon, A. G. Kaditis, D. Splaingard, M. Splaingard, L. J. Brooks, C. L. Marcus, S. Sin, R. Arens, S. L. Verhulst, and D. Gozal, “Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study,” Am. J. Respir. Crit. Care Med., vol. 182, no. 5, pp. 676–683, Sept. 2010.
- W. E. Schaaf, C. T. Wootten, L. F. Donnelly, J. Ying, and S. R. Shott, “Findings on MR sleep studies as biomarkers to predict outcome of genioglossus advancement in the treatment of obstructive sleep apnea in children and young adults,” AJR Am. J. Roentgenol., vol. 194, no. 5, pp. 1204–1209, May 2010.
- R. Arens, J. M. McDonough, a T. Costarino, S. Mahboubi, C. E. Tayag-Kier, G. Maislin, R. J. Schwab, and A. I. Pack, “Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome,” Am. J. Respir. Crit. Care Med., vol. 164, no. 4, pp. 698–703, Aug. 2001.
- R. Schwab, M. Pasirstein, R. Pierson, A. Mackley, R. Hachadoorian, R. Arens, G. Maislin, and A. Pack, “Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging,” Am. J. Respir. Crit. Care Med., vol. 168, no. 5, pp. 522–530, Sept. 2003.
- L. F. Donnelly, S. R. Shott, C. R. Larose, B. A. Chini, and R. S. Amin, “Causes of persistent obstructive sleep apnea despite previous tonsillectomy and adenoidectomy in children with down syndrome,” AJR Am. J. Roentgenol., vol. 183, no. 1, pp. 175–181, July 2004.
- M. E. Wagshul, S. Sin, M. L. Lipton, K. Shifteh, and R. Arens, “Novel retrospective, respiratory-gating method enables 3D, high resolution, dynamic imaging of the upper airway during tidal breathing,” Magn. Reson. Med., vol. 70, no. 6, pp. 1580–1590, Dec. 2013.
- I. M. Colrain, K. Nayak, and J. Nielsen, “Real-time MRI of upper airway collapse during inspiratory loading,” in Proc. ISMRM, 2006, p. 2417.
- Y. Kim, R. M. Lebel, Z. Wu, S. L. D. Ward, C. K. Khoo, and K. S. Nayak, “Real-time 3D magnetic resonance imaging of the pharyngeal airway in sleep apnea,” Magn Reson Med., vol. 71, no. 4, pp. 1501–1510, Apr. 2014.
- Y.-C. Kim, B. Joshi, S. Loloyan, R. Kato, M. C. K. Khoo, S. L. D. Ward, and K. S. Nayak, “Investigations of upper airway obstruction pattern in sleep apnea benefit from real-time 3D MRI,” in Proc. ISMRM 2014, p. 4387.
- Z. Wu, Y. Kim, M. C. K. Khoo, and K. S. Nayak, “Novel upper airway compliance measurement using dynamic golden-angle radial FLASH,” in Proc. ISMRM 2014, p. 4323.
- G. Mylavarapu, M. Mihaescu, L. Fuchs, G. Papatziamos, and E. Gutmark, “Planning human upper airway surgery using computational fluid dynamics,” J. Biomech., vol. 46, no. 12, pp. 1979–86, Aug. 2013.
- D. Idiyatullin, C. Corum, J. Y. Park, and M. Garwood, “Fast and quiet MRI using a swept radiofrequency,” J. Magn. Reson. vol. 181, no. 2, pp. 342–349, July 2006.