Above: Examples of 3-D-printed prototypes of the PDMonitor wearable devices. (Image courtesy of PD Neurotechnology Ltd.)
PD Neurotechnology specializes in an innovative medical device, the PDMonitor, designed to assist patients with Parkinson’s disease (PD). The company was founded by four diverse people:
- Dimitrios Fotiadis, an academic and expert in biomedical informatics
- Spyridon Konitsiotis, a distinguished neurologist specializing in PD
- Nikolaos Moschos, a seasoned business manager experienced in the healthcare sector
- Anastasios Manos, a strategic planning and vendor management professional
Funding has been raised from private angel investors, and further funding has been awarded by the National Bank of Greece through the Business Seeds competition for innovation and technology. PD Neurotechnology has already acquired International Standards Organization (ISO) 9001, ISO 27001, and ISO 13485 certifications and is currently running three clinical trials (in Greece, Italy, and Germany) to validate the wearability, usability, accuracy, and safety of the PDMonitor so as to obtain the European Union’s medical CE mark.
IEEE Pulse recently spoke with Nicholas Kostikis (pictured below), Ph.D. degree candidate with the Department of Applied Informatics, University of Macedonia, who works on the company’s research and development team, about its motivation for tackling movement disorders.
Ahmed Morsy: Why did you choose to focus on PD?

Nicholas Kostikis: PD is a neurodegenerative, chronic disease. It affects more than 1% of people over 55 and 3% of people over 75 years of age. According to the Parkinson’s Foundation, that translates to more than 10 million people worldwide, with approximately 60,000 Americans being diagnosed each year. PD incidence increases with age, which means that we are about to have more patients as life expectancy increases.
More than 4% of people diagnosed with PD are actually younger than 50 years of age, and most of them will live with their disease for two or more decades. During the early years of PD, the patients respond well to the dopaminergic medication, and the visits to their treating physician can be sparse. However, as the disease progresses, the response to the medication deteriorates, and the patients suffer from fluctuations and side effects. During fluctuations, the patients start to feel their symptoms much earlier than the next scheduled medication intake, which can be rather frustrating and demoralizing. To make matters worse, side effects, namely, dyskinesias, can be so prominent that most people usually mistake druginduced dyskinesias as PD cardinal symptoms.
AM: What market gap was PD Neurotechnology founded to fill?
NK: As I mentioned before, as the disease progresses, PD patients suffer from severe medication side effects. Also, the response to their medication deteriorates, and their medication starts wearing off at irregular and seemingly random intervals, leaving them with symptoms well before their next scheduled dose. However, these unfortunate effects would not be particularly detrimental for the patient’s daily living if their treating physicians could be made aware of each patient’s situation and respond (in a timely manner) to address the symptoms. However, it is typical for PD patients to schedule an appointment with their physician many months later than the onset of dyskinesias or wearing off of symptoms.
When that appointment actually takes place, the treating physicians rely heavily on patients’ testimony and diaries to adjust the medication regimen. During a routine half-hour clinical examination, it is very difficult for the healthcare professional to observe the patient’s disease symptoms and prescribe the correct adjustments in timings and dosages to optimize symptom alleviation.
In chronic diseases, patient–doctor interaction and communication are crucial. Patients need to feel confident that their treating physician is close to them and that when they feel discomfort, they will be able to reach her or him and get advice that will actually help. In the traditional clinical practice, physicians often lack actionable knowledge on patients’ symptoms, missing critical parts of the puzzle such as actual symptom severity, eating habits, activity schedule, and minor but relevant deviations. Later-stage PD patients require more frequent periodical medication titration to feel their best, which can be costly and cumbersome, particularly in rural areas, where access to trained and experienced healthcare professionals can be more difficult.
The PDMonitor is a noninvasive, continuous home-monitoring system, built to be used by patients with PD. Its intended use is to record patient activity and movement patterns in a long-term fashion throughout the day and provide actionable knowledge to the treating physician on a daily basis. This is an entirely new treatment protocol, and, currently, there is no established market for a product like PDMonitor and the service it can offer. PD Neurotechnology aims to disrupt the current paradigm of chronic disease management and empower patients (who will be the principal focus in a subscription-based treatment protocol) to own their data, transfer them at will, and offer their potential treating physicians a full history of their disease progression, medication regimens, and important disease-related events.
AM: What is the nature of the product technology?
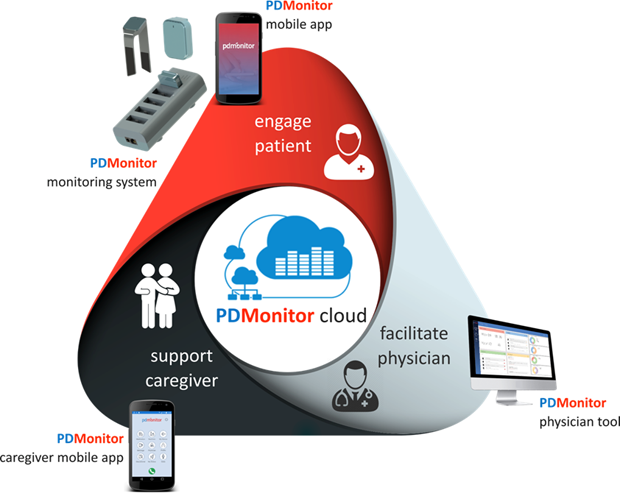
PDMonitor is a noninvasive, continuous home-monitoring system intended to identify, record, process, and quantify a variety of motor and nonmotor manifestations of PD. It consists of
- a set of wearable monitoring devices and their docking station (pictured at the top of this article)
- a mobile application that enables patients and caregivers to record medication, nutrition, and nonmotor status as complementary information for the motor symptom assessment
- a web-reporting application, the Physician Reporting Tool, which offers to the healthcare professional comprehensive graphical representations of all patient-related information.
The system provides a closed loop of interaction among the patient, the caregiver, and the healthcare professional (above) and, at the same time, a repository of longitudinal patient health status-related data.
Movement information derived from the recordings is translated to disease symptoms with correlated intensities. In the graphical representations, other information such as nutrition, actual medication intake (as it is recorded by the patient), and activity can optionally be superimposed onto the graphs to provide actionable insights for the healthcare professional and help in acquiring a deep understanding of the patient’s fluctuations and symptom severity.

AM: How did you make the shift from research to entrepreneurship? How hard was that transition?
NK: For many years, research has been a side project for me because I wasn’t wellfunded and I was always working as a professional software engineering instructor or a software developer. It was a blessing for me to actually put everything else on hold and be dedicated to building a medical product, which would heavily rely on research but also be developed according to the highest standards and requirements.
AM: What is the biggest challenge you are facing now?
NK: The one thing I wasn’t fully prepared for was the huge time and effort required to follow the processes and prepare the documents necessary for regulatory compliance. Everyone on our team was more or less experienced with research, innovation, the software product development life cycle, and verification and validation methods. But to actually put this in a quality management system, applying the traceability rules and complying with all the regulations and standards required by the European Union’s MEDDEV directives and its Medical Device Directive means long hours and real dedication.
AM: What is the one thing that, if made available, would help this startup succeed?
NK: Like all startups, we could always use more funds and time. Lack of either can be detrimental to a startup’s success. Hasty decisions and cost reductions when building a medical product can lead to failure. Thankfully, within our team there is no shortage of talent, and PD Neurotechnology is supported by business and financial managers and advisors experienced enough to steer the company through the difficulties.
AM: What kind of advice would you give to any researcher turning into entrepreneur?
NK: There is one thing that every researcher needs to keep in mind when aspiring to commercialize her or his fruit of labor: evidence. Far too many great innovative ideas will never get past the lab and into the market due to lack of tangible evidence that the actual product could be sufficiently and sustainably effective. In my mind, the path to further rounds of funding and a successful exit or production goes through evidence and regulatory compliance.



