Nuclear medicine has come a long way in a short time. Over the past three decades alone, it has taken two major steps forward and is now on the precipice of yet another advance that could begin to have a real impact on cancer care within the year.
One of those major steps came in the 1990s when FDG-PET, or positron emission tomography with 18-fluorode-oxyglucose (18FDG), took on a starring role in the clinic, especially as it relates to oncology. FDG-PET exploits the fact that cancer cells grow quickly and are very active metabolically, and consequently, they are glucose gluttons. When a radioactive analog of glucose is administered (in the form of 18FDG, or glucose tagged with radioactive fluorine), tumor cells metabolize it. PET then picks up the radioactivity, thereby imaging cancerous sites. Clinicians quickly saw its value for detecting cancer and have since made it a standard of care for tracking cancer progression and evaluating whether a given treatment is having the desired effect.
In the early 2000s, the field surged ahead again with the advent of hybrid imaging for clinical use. Perhaps the most popular is PET/CT, which combines PET with computed tomography (CT), an imaging technique that uses X-rays to provide cross-sectional images of anatomical structures. Other hybrid techniques include such options as SPECT/CT and PET/MRI. The former incorporates single photon emission CT (SPECT), which employs a gamma camera and a radioactive tracer to provide three-dimensional images of blood flow, brain activity, or other functions; and the latter incorporates magnetic resonance imaging (MRI), which provides cross-sectional views of anatomical structures but utilizes magnetic fields and radio waves to generate images. Hybrid imaging options like these allow the nuclear medicine physician or radiologist access to a wide range of information that can help direct patient care and, in many cases, may permit the identification of an abnormality that would be missed if only a single imaging technique were used.
Doing It All with Theranostics
Today, nuclear imaging is entering another new era, one that combines not only the diagnosis and tracking of cancer, but also the delivery of cancer treatment. Dubbed theranostics, this multi-pronged approach is made possible by new radiopharmaceuticals that bind to tumor cells, penetrate them, and kill them. These highly specific and very effective agents are also designed to target some of the most challenging cancers, such as gastroenteropancreatic neuroendocrine tumors, which are difficult to diagnose and treat, and metastatic castration-resistant prostate tumors, which often have few if any treatment options available.
“I think theranostics is the most striking example of precision medicine, because you use nuclear imaging to determine whether the therapeutic target is expressed or present, which allows you to predict whether a patient will respond or not respond, and then you use the same molecule but with a different label to address your therapeutic target,” says Johannes Czernin, M.D., chief of the University of California at Los Angeles (UCLA) Ahmanson Translational Imaging Division (Figure 1). “For instance, this gives a patient with prostate cancer considerable hope for extended, progression-free, and overt survival, and it gives them clearly and without any doubt and we know this already marked symptomatic improvement.” He continues, “Theranostics is something that will be extremely successful over the next ten years, and it will cement the role of nuclear medicine as an independent discipline.”

Folate as a Stepping Stone
For many researchers, the modern rise of nuclear medicine can be traced to the B-vitamin folic acid, or folate. Folate came to the forefront following the early-1990s discovery by Purdue University researchers that it could be used to home in on a wide range of cancer cells that happen to be coated with folate receptor proteins. In addition, folate analogs could be developed to carry other molecules to those cells [1], [2]. Building on this work, research groups began developing targeted cancer-imaging agents that combine folate with short-lived radioactive tracers for use in both SPECT and PET imaging platforms. Early studies showed that, by injecting these agents into tumor-bearing animals, the folate-targeted radio-ligands travel throughout the body and specifically bind to the folate receptors expressed on cancer cells. SPECT or PET scanning could then pick up the radiation to anatomically image the tumor in real time.

Less than a decade later, the spinoff company Endocyte, Inc., of West Lafayette, Indiana, was launched with the intent of taking the Purdue work to the next level, says Christopher P. Leamon, Ph.D., vice president of research and development at Endocyte (Figure 2, right: Image courtesy of Endocyte, Inc.). As a doctoral student, Leamon was a key member of the pioneering Purdue research group under Philip S. Low, Ph.D. Low helped found Endocyte in 1996 and currently serves as its chief science officer.
The company has invested time developing folic acid radio-ligands to use with SPECT/CT as well as PET/CT. For instance, one of Endocyte’s assets, EC20, is a folic acid derivative that chelates the radionuclide 99mTechnetium (99mTc). Approximately an hour after a patient is injected with 99mTc-EC20, SPECT-CT is employed to image the captured radioactivity and reveal whether a folate receptor-positive tumor is present in the body.
Focus on Prostate Cancer
Today, research groups (including Endocyte) are exploring the potential not only to image tumors but also to treat them. A major focus of these projects has been on prostate-specific membrane antigen (PSMA). The interest in PSMA stems from its presence in more than three-quarters of patients who have metastatic castration-resistant prostate tumors. This type of incurable cancer has very limited treatment options, and it typically manifests in the skeleton, where it of-ten causes considerable bone pain.
Endocyte began studying PSMA around 2005, and it has since developed a SPECT-based radioactive imaging agent, 99mTc-EC0652, as well as a companion tubulysin-based small-molecule drug conjugate, EC1169, to attack the tumor cells. Other research groups have also taken up the challenge, including one at the German Cancer Research Center and University Hospital Heidelberg. That group developed a compound called PSMA-617 and eventually licensed it to the German chemical company ABX to produce the agent in support of early clinical development. In those trials, participants received the radioligand therapeutic 177Lu-PSMA-617 (Figure 3), which is PSMA-617 labeled with the short-range beta-emitting radionuclide lutetium-177 [3].
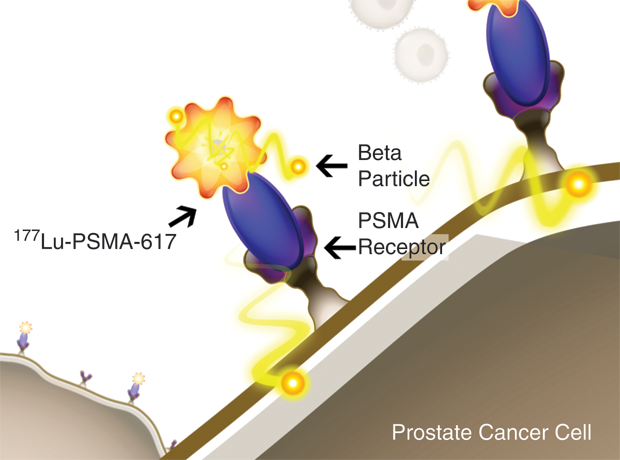
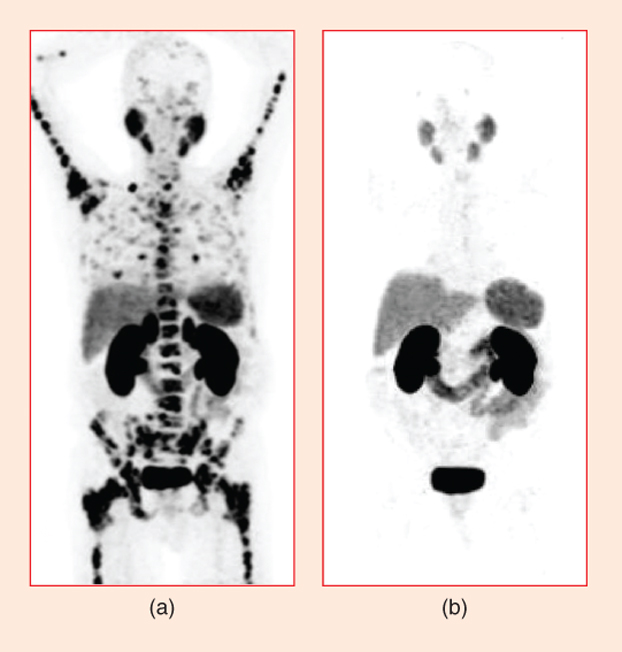
“The initial idea with 177Lu-PSMA-617 was to treat prostate cancer patients who had essentially exhausted all of their therapeutic options,” Leamon explains. “It has since been tested in hundreds of patients around the world, and, in every trial, it has been shown to be quite active” (Figure 4). The treatment’s success drew Endocyte’s attention, he says, and, in October 2017, Endocyte announced that it had obtained exclusive global rights from ABX to develop and commercialize the highly promising PSMA-617 agent [4].
The pairing of 177Lu with PSMA-617 is especially effective for several reasons, according to Leamon. “In addition to PSMA-617 having a high specificity for prostate tumor cells,” he says, “it is a small molecular weight compound capable of penetrating large solid tumors, including widely disseminating prostate-cancer tumors. Further, its destructive range is no more than 2 mm, which results in minimal damage to surrounding healthy tissue.”
Leamon projects that Endocyte will begin phase 3 clinical trials of 177Lu-PSMA-617 for use in metastatic prostate cancer during the first half of 2018, with results following by 2020. In the meantime, Endocyte will look into the possibility of using the agent for other cancers. “To some extent, the PSMA target is also present in the newly forming blood vessels of many other cancers,” he says, so the company will eventually be looking into potential uses of PSMA-617 beyond prostate cancer.
In addition to folate and PSMA, Endocyte has a variety of small ligand-based technologies and also plans to expand its internal radioligand development program. “Radioligand therapies, or RLTs, are the next wave of new cancer discoveries leading to improved therapies for patients. We’re excited to be at the forefront of this new technology,” Leamon notes, adding: “Stay tuned, because Endocyte may have other RLTs coming out in the future.”
The Gallium Connection
Gallium (Ga)-labeled radionuclides are also under study for staging cancers and selecting patients for RLTs. That includes the efforts of Thomas Hope, M.D. (Figure 5), an assistant professor of abdominal imaging and nuclear medicine at the University of California at San Francisco (UCSF), who has been evaluating Ga compounds for use in both neuroendocrine tumors and prostate cancer.
Neuroendocrine tumors arise from hormone-secreting precursor cells and can originate in many parts of the body, including the colon, stomach, pancreas, and lungs. Approximately 90% of these tumors express a receptor for the hormone somatostatin on tumor-cell surfaces. This presents a target for researchers, who have developed somatostatin analogs labeled with the radioactive metal Ga-68 (68Ga), Hope explains. “A PET/CT detects the small amount of radiation attached to these molecules, and this allows us to see small sites of disease with very high sensitivity and specificity.” That’s important, he says, because “this is the most effective way to image these tumors.”
The success of 68Ga-labeled somatostatin analogs led researchers to take the approach a step further: they began replacing 68Ga with therapeutic radiation: 177Lu and yttrium-90. This approach is also known as peptide receptor radionuclide therapy, or PRRT. In January 2017, the results of a large, randomized phase 3 trial, NETTER-1 [5], demonstrated markedly prolonged, progression free survival in patients with midgut neuroendocrine tumors. While the agent, called 177Lu-DOTATATE, or Lutathera, is not curative, it prevents disease progression and can alleviate symptoms in patients with functional tumors.
The U.S. Food and Drug Administration (FDA) is expected to approve 177Lu-DOTATATE early this year, and, once that happens, it should begin to become available to patients soon thereafter, Hope says. “Lutetium DOTATATE will have a huge impact, both for patients as well as for the field of nuclear medicine, because it will help bring nuclear medicine to the table with medical oncologists participating in the management of a patient’s care.”
Hope is also leading a large U.S. trial to study another compound and its efficacy against prostate cancer. Called 68Ga-PSMA-11, it is a compound developed by researchers at the University of Heidelberg and the Technical University of Munich [6]. Like the PSMA-617 under study by Endocyte, PSMA-11 binds to PSMA-expressing tumor cells. UCSF was the first site in the United States to start using PSMA-11, partly because it already had the infrastructure in place to study Ga-containing agents, and its clinical trial of 68Ga-PSMA-11 is well under way [6]. “We’ve gone after it fairly aggressively, and we have 800 patients in our clinical trial using that imaging agent,” Hope says. “Hopefully, we now have accumulated enough clinical data to go to the FDA in the coming year and get New Drug Application approval for this therapeutic agent.”
Perfect Pairing
With the emergence of more and more RLTs, the door to a new age of nuclear medicine is beginning to open, Czernin says. “The approval of lutetium-labeled DOTATATE for neuroendocrine tumors in [early 2018] and [current work on] theranostic pairings of gallium-labeled and lutetium-labeled PSMA ligands for prostate cancer are completely changing the practice of nuclear medicine,” he stresses. “These treatments are going to be a big deal for patients and practitioners, because there will suddenly be something in hand that can be quite effective symptomatically.”
Besides participating in some of the RLT trials, Czernin and his research group are also using both PET and optical imaging, as well as mass spectrometry, to detect exactly how tumor cells are responding metabolically to innovative radiopharmaceuticals. “This helps us to find out how these resistance mechanisms emerge and how they can be attacked,” he says, noting that such information is crucial for defining new theranostic targets and approaches.
In addition, he says that UCLA is starting to build an outpatient clinic where theranostics will be available. “Several other places are also going to put in theranostics centers that are solely focused on treating patients with RLT and are run by nuclear medicine professionals, while, at the same time, are highly collaborative with the clinical practice of such fields as urology, oncology, endocrinology, and other disciplines,” Czernin elaborates.
Demand for RLT is already high, so the centers cannot come soon enough, he continues. “My phone is ringing off the hook here with patients wanting to know when they can get the treatment, and urologists are calling us to find out when we are finally ready to go.” Based on projections, Czernin foresees need for 100–150 theranostics centers throughout the United States. “I don’t know that we at UCLA will be the first center, and I don’t care much if we are first or not. What is important to me is that we start to have these centers,” he remarks. Once a center is open, he says, it will gather evidence to show RLTs’ benefit, and theranostics will become a significant tool in the fight against cancer.
Neuroendocrine and prostate tumors are just the beginning for RLTs, adds Hope. “There are other therapies being developed, but they are much farther off. These include therapies for multiple myeloma and lymphoma, and then there is the possibility of using an alpha emitter, such as bismuth or actinium.” In summary, he says simply, “RLTs are going to be a big deal.”
Czernin agrees. “Theranostics is a tremendous opportunity for patients, for physicians, and for the field of nuclear medicine.”
References
- C. P. Leamon and P. S. Low, “Delivery of macromolecules into living cells: A method that exploits folate receptor endocytosis,” Proc. Nat. Acad. Sci. U.S.A, vol. 88, no. 13, pp. 5572–5576, 1991.
- I. R. Vlahov and C. P. Leamon, “Engineering folate-drug conjugates to target cancer: From chemistry to clinic,” Bioconjugate Chem., vol. 23, no. 7, pp. 1357–1369, 2012.
- A. Bräuer, L. S. Grubert, W. Roll, A. J. Schrader, M. Schäfers, M. Bögemann, and K. Rahbar, “177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer,” Euro. J. Nucl. Med. Molec. Imaging, vol. 44, no. 10, pp. 1663–1670, 2017.
- Endocyte Inc. (2017, Oct. 2). Endocyte announces exclusive worldwide license of phase 3 ready PSMA-targeted radioligand therapy for development in prostate cancer. [Online].
- J. Strosberg, G. El-Haddad, E. Wolin, A. Hendifar, J. Yao, B. Chasen, E. Mittra, P. L. Kunz, M. H. Kulke, H. Jacene, D. Bushnell, T. M. O’Dorisio, R. P. Baum, H. R. Kulkarni, M. Caplin, R. Lebtahi, T. Hobday, E. Delpassand, E. Van Cutsem, A. Benson, R. Srirajaskanthan, M. Pavel, J. Mora, J. Berlin, E. Grande, N. Reed, E. Seregni, K. Öberg, M. L. Sierra, P. Santoro, T. Thevenet, J. L. Erion, P. Ruszniewski, D. Kwekkeboom, and E. Krenning, “Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors,” N. Eng. J. Med., vol. 376, no. 2, pp. 125–135, 2017.
- M. Eder, M. Schäfer, U. Bauder-Wüst, W.-E. Hull, C. Wängler, W. Mier, U. Haberkorn, and M. Eisenhut, “68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging,” Bioconjugate Chem., vol. 23, no. 4, pp. 688–697, 2012.