New technology offers help for failing discs in efforts to reduce pain and restore function
The World Health Organization calls low back pain the single leading cause of disability worldwide, and one that can lead to limitations in activities and erosion of the quality of life [1]. The pain is often caused by degeneration of one or more shock-absorbing intervertebral discs, each of which is essentially a collagen-and-water cushion, called nucleus pulposus (NP), that lies within a strong, collagen-rich sack, known as the annulus fibrosis.
Treatment recommendations for this condition—degenerative disc disease (DDD)—typically involve taking anti-inflammatories, trying physical therapy, and retuning the diet. While they may help lessen symptoms, they do not address the core issue: the degraded disc. Now, however, a number of research groups are taking aim at the disc with noninvasive, injectable treatments.
Examples of the options under development include:
- a gel that fills and seals a damaged disc, by
ReGelTec, Baltimore, MD, USA; - a protein-based biologic, by Notogen, Toronto, ON, Canada; and
- a cell therapy that incorporates “disc-derived” differentiated progenitor cells, by DiscGenics, Salt Lake City, UT, USA.
The three options work differently but share the same objective of reducing pain and restoring function for people with DDD. ReGelTec hopes to help patients with moderate to severe disease, while Notogen and DiscGenics are targeting those with mild to moderate disc degeneration.
Gel implant
ReGelTec’s product, called HYDRAFIL, is a gel designed to both fill the NP space as well as tears in the annulus fibrosis that can become particularly prominent in severe disease. “It restores some of the normal loading to the disc, and because it has a hydrophilic nature, it also brings back water and rehydrates the disc somewhat,” explained Tony Lowman, professor of BME at Rowan University, Glassboro, NJ, USA, and co-founder and chief technology officer of ReGelTec (Figure 1).

Figure 1. Tony Lowman, professor of BME at Rowan University, Glassboro, NJ, USA, and co-founder and chief technology officer of ReGelTec, which developed HYDRAFIL to help patients experiencing low back pain. (Photo courtesy of Rowan University.)
HYDRAFIL is an outgrowth of Lowman’s earlier work, which initially focused on a solid gel implant. “Our approach then was to completely refill the disc, so it would be exactly the way it was prior to degeneration,” he said. To further develop the idea, he formed a small startup called Geliflex, and later sold it to the medical manufacturer Synthes Inc. in the mid-2000s. “They ran some trials in Europe, but it wasn’t successful,” he recalled.
Lowman took everything he learned from that first attempt and shifted his focus away from a solid gel implant, and toward an injectable liquid gel that would augment the existing NP instead of replacing it. “The idea was that we wouldn’t make the patient ‘perfect’ again, but we would definitely reduce pain and improve quality of life,” he said.
In 2018, Lowman co-founded ReGelTec to help develop the injectable liquid gel. To do it, researchers in his group at Rowan and ReGelTec modified the solid gel’s formulation, added a new component, and focused on the processing stage in order to produce a material that could be heated to a liquid state for easy injection, but would then cool to a solid once it was in place in the disc (Figure 2).

Figure 2. HYDRAFIL is a material that is heated to a liquid state for easy injection (pressure gauge and the 17 gauge needle shown here) into the NP of the degenerated disc. The material cools to a solid once it is in place. (Image courtesy of ReGelTec.)
“We took advantage of the different interactions between polymers, which mostly consisted of polyvinyl alcohol, and water to get physical crosslinks to form and generate the gel in the body,” he explained. “So based on what we know about polymer thermodynamics, how things crystallize, how things are miscible or not miscible, and putting all of those different aspects together, we were able to get very similar properties (to the solid gel) in terms of biocompatibility, water absorption, and mechanical properties, but we could place it by just using a needle through a very small hole, which is then sealed up by the gel itself,” he described.
Once they had advanced the prototype sufficiently, they started preclinical testing in animal models, established feasibility, and then began talks with the U.S. Food and Drug Administration (FDA) about developing the HYDRAFIL gel for human use, while also exploring clinical testing in other countries (Figure 3). In the first quarter of 2020, they received approval to begin testing in Colombia, and they were ready to start in April, but the pandemic forced a delay. When the trial finally commenced in August 2020, ReGelTec’s U.S. team could still only proctor via Zoom due to pandemic-related restrictions. “I actually watched that first patient be successfully treated from a parking lot in Niagara Falls, which was the only place my family could get away for a vacation,” Lowman remembered.
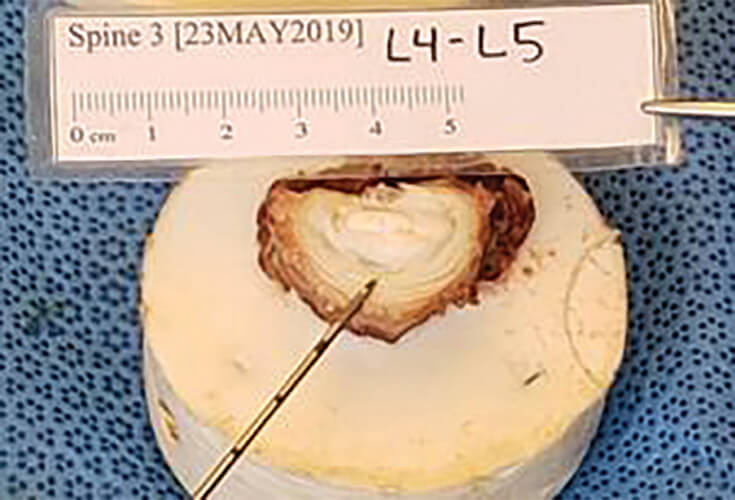
Figure 3. In animal/cadaver studies, ReGelTec researchers were able to dissect the disc following treatment with HYDRAFIL and see the material in the NP. (Photo courtesy of ReGelTec.)
After a dozen patients had been successfully treated in Colombia via Zoom, the U.S. ReGelTec team obtained permission to enter Colombia and participate in-person. In total, researchers enrolled 20 patients with chronic low back pain, employed fluoroscopic imaging to inject HYDRAFIL directly into the affected discs, and measured patient response. They reported that HYDRAFIL improved average pain-scale scores from 7.1 to 2.0 at 6 months posttreatment [2].
Since then, they have treated additional patients in Colombia and in a trial in Calgary, Canada, with good results. Among patients treated with HYDRAFIL with 1-year follow-up data (N = 58), the average pain score on a scale of 0–10 dropped from 7.2 to 2.2, and down to 1.8 at the two-year point (N = 17), Lowman said. When analyzed with the Oswestry Disability Index (a measure of the effect of pain on quality of life), patients averaged a score of 58.6 pretreatment, which falls in the “severe disability” range, and 11–12 posttreatment, which is in the “mild disability” range at both the one-year and two-year marks [3].
At this stage, Lowman is unsure how long the injections will continue to provide relief for patients. “We’ve done fatigue studies where we’ve run these gel materials out 10 million cycles, or to what is like a 40–50-year assessment, and there’s no indication they’re breaking down. So, if HYDRAFIL lasts three years in a patient, that would be good; five years would be very, very good; and 10 would be exceptional,” he said. “And one of the nice things about this gel is we don’t burn any bridges, so if a patient eventually needs a different procedure later on, it’s easy to remove the gel through a very simple endoscopic technique if necessary.”
ReGelTec received breakthrough device designation from the FDA in 2020, and recently received FDA investigational device exemption (IDE) approval to start a 225-patient pivotal study in the U.S. “We’re very excited to begin an FDA study because they’re a tough regulatory body, but also the gold standard,” Lowman said. “All of our data so far points to us being successful in an FDA study, so this could be a treatment not too far down the road for people who don’t really have any other good options today.”
Protein-based biologic
Notogen’s protein-based biologic, named NTG-101, got its start when then-graduate student W. Mark Erwin heard about the work of Theodore Oegema at Rush University Medical Center, Chicago, IL, USA, involving certain chondrodystrophic dog breeds, such as basset hounds, dachshunds, and beagles, that are especially prone to DDD. In the late 1980s, Oegema’s group found that cell culture seeded with notochordal cells from the discs of nonchondrodystrophic dogs, stimulated the disc cells to produce proteoglycans, which are key components of the connective tissue found in intervertebral discs [4]. “That’s all they knew at that point. They didn’t know why or how it happened,” said Erwin, D.C., Ph.D., assistant professor of neurological and orthopedic surgery at the University of Toronto, Scarborough, ON, Canada (Figure 4).

Figure 4. W. Mark Erwin of the University of Toronto and the start-up Notogen are developing a protein-based biologic, named NTG-101, which incorporates TGF-β1 and CTGF as a means to help restore degenerated discs. (Photo courtesy of W. Mark Erwin.)
Erwin was so intrigued that he changed his graduate studies focus to try to decipher how these “fascinating notochordal cells could possibly protect the disc from undergoing degeneration.” This pursuit became his focus throughout graduate school and post-doctoral research, all of which led to the 2016 formation of the Notogen start-up. “We found that notochordal cell-conditioned media increased the expression of important genes that control disc health, suppressed disc cell death, and in a rodent model of disc disease, clearly enhanced repair post-injury. The results of these experiments convinced me that a soluble, secreted family of proteins might be conferring a protective effect upon the disc cells and milieu, and that could maybe be harnessed as an injectable therapeutic someday,” he said. The path has taken Erwin and Notogen researchers through a series of “complicated molecular, proteomic, and bioinformatic” experiments—both in vitro and in vivo—that narrowed their focus to two notochordal cell-secreted proteins: transforming growth factor beta 1 (TGF-β1) and connective tissue growth factor (CTGF).
In 2018, the researchers reported that they had developed the biologic NTG-101, which incorporated TGF-β1 and CTGF in an excipient solution, and had tested a single injection of it using in vivo models of DDD, including in chondrodystrophic canines [5]. The canine study included 16 dogs with three injured discs per dog. Six of the dogs each received a saline injection in three discs per animal, and 10 received an injection of NTG-101 also in three discs per animal. “We waited 14 weeks postinjection, and found through X-ray, magnetic resonance imaging (MRI), molecular, histological and biomechanical analyses that injured discs injected with the drug are virtually indistinguishable from normal discs. And we clearly saw increased proteoglycans and collagen type II, and suppression of all the catabolic enzymes that accompany DDD,” Erwin said. “It was a home run.”
Following up, the researchers examined the disc samples for signatures that would indicate changes in pain levels in the dogs [6]. “It’s been shown that humans strongly express certain neurotrophins and neuropeptides (e.g., brain-derived neurotrophic factor, nerve growth factor receptor, and others) that are associated with pain, so we looked for those in the dog discs,” Erwin said. Their results showed that among injured dog discs, those injected with saline experienced skyrocketing levels of neurotrophins and neuropeptides, but those injected with NTG-101 were the same as normal discs.
Erwin said acquiring funding and an industrial partner to help move NTG-101 from the lab to the clinic had been a bit of a challenge, but with the support of a private investor and a recent collaborative research agreement with an industrial partner, the product is on a strong forward path (Figure 5). “If things go well over the near-term, the idea will be to license all of our technology to our partner, and from there to move through clinical trials,” he said.

Figure 5. With extensive analyses and experimentation of NTG-101 showing good results, Erwin looks forward to licensing the Notogen technology to its industrial partner, and from there, to beginning clinical trials. (Photo courtesy of W. Mark Erwin.)
Although Erwin would like to see the product make it to the clinic as soon as possible, slow and steady is the best approach to ensure efficacy and safety, and to define the optimal patient candidates for the NTG-101 therapeutic, he said. “I think this offers great promise. I really do. After all these years, I am still pursuing it because the underlying science is so compelling.” Others apparently think so, too. He noted, “We’ve won best paper at North American Spine Society meetings twice, published in good journals, and all of our work is open to scrutiny, so I am quite confident that we are on a good track, and this may be a really novel molecular therapy.”
Disc-derived progenitor cells
DiscGenics is taking an alternate line of attack. It is developing IDCT (an acronym for injectable disc cell therapy) that combines approximately 9 million differentiated progenitor cells, which the company calls discogenic cells, with a proprietary viscous scaffold carrier that both protects the cells and helps them disperse within the disc (Figure 6). One of the benefits of using differentiated progenitor cells over stem cells is their far longer-lasting effects, according to Bob Wynalek, the company’s chief operating officer. “Some groups have taken mesenchymal stem cells and put them into the disc, but they aren’t regenerative, so while they are immunomodulatory, which can reduce pain and inflammation, they are not durable long-term.”
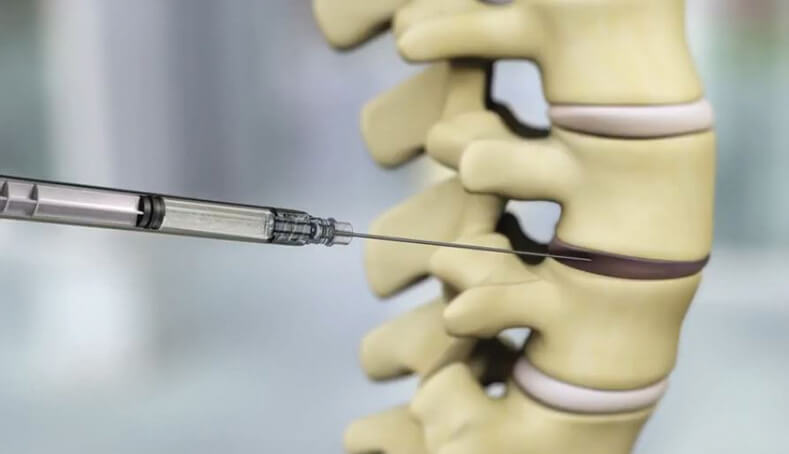
Figure 6. DiscGenics’ IDCT, shown being injected into a degenerated disc, combines millions of differentiated progenitor cells, called “discogenic cells,” with a proprietary viscous scaffold carrier. The company preparing to accept patients into its phase III clinical trial of high-dose IDCT, slated to begin in mid-2024. (Photo courtesy of DiscGenics.)
For their approach, DiscGenics researchers start with donor-derived disc cells, and put them through an approximately two-month, cell-culture process to differentiate them into discogenic cells. “Because they’re differentiated, they’re already producing disc-matrix components, such as collagen and proteoglycans,” Wynalek said.
Proteoglycans are of special note, he remarked. “When the degenerated disc disease progress starts, the extracellular matrix in the NP breaks down and the proteoglycans go away, but it is the proteoglycans that hold in the necessary hydration.” Therefore, as proteoglycans disappear, disc cushioning lessens, discs bulge, the vertebral column has more movement, and can lead to pressure on surrounding nerve roots, and in turn, cause low-back pain and potentially numbness and pain that runs down the leg, he said. “By rebuilding the extracellular matrix with discogenic cells, however, a dose of IDCT helps mechanically restore and stabilize the disc, while also serving as an immunomodulatory and anti-inflammatory therapy.”
DiscGenics has already conducted studies in small and large animals (rabbits and mini-pigs), and in humans, and have reported positive results [7], [8]. “The cells act very quickly,” Wynalek said. “As soon as four weeks (postinjection) in treated animals, we had restored disc height to within about 80% of normal. And in our human trials, we saw significant improvement in all the patient-reported outcome measures beginning at 12 weeks, including the visual analog scale (VAS) for pain, the Oswestry Disability Index, and the European Quality of Life Survey, and we also saw statistically significant improvements in disc volume.” Studies of high-dose IDCT show durability of those results out to two years, he recounted.
IDCT received the FDA’s fast track designation in August 2019 and a regenerative medicine advanced therapeutic (RMAT) designation in January 2023. The latter gives the company heightened interaction with the FDA and accelerated review, which will hopefully speed agency approval, Wynalek said. DiscGenics is also in similar discussions with Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), he added, noting that either an FDA or PDMA approval will be useful for doing bridging studies and obtaining approvals in other countries as well.
The company is now preparing to accept patients into its phase III clinical trial of high-dose IDCT, which will begin in mid-2024, and is working toward doing its own late-clinical and commercial manufacturing, which the company believes will allow for greater quality control and more profitability, he said. In fact, DiscGenics is now in the final stages of validation at its new manufacturing facility with plans to start up late-clinical-stage manufacturing in late 2023 or early 2024, Wynalek said. If all goes well, he expects IDCT to make it to the market as early as 2027.
How about other joints?
One of the questions that low-back researchers often hear is whether their technologies could also be applied to other areas. For Notogen, the query regularly centers on treatments for back issues in chondrodystrophic dog breeds. “It makes sense to me that this will be a veterinary product at some point,” Erwin said. “In fact, this is something that would definitely be attractive to us down the road.”
DiscGenics is likewise keeping its attention on the lumbar spine, and once that is squared away, will begin looking further, Wynalek said. “It’s on our radar to look at broader musculoskeletal applications, and we certainly have done some initial feasibility studies on possible treatment for arthritis, but obviously our primary focus is getting lower-back IDCT approved and generating revenue. Then, we can pour more investment into some of these additional research and development programs.”
At ReGelTec, Lowman said he frequently fields questions about applying the hydrogel to other joints. “Everyone asks, ‘Can I use this in my knee?’ or ‘Can I use it in my shoulder?’ And the answer is, no, not with this particular version as it is developed. But we have other formulations that we think could work,” he said. “And once we get started in our clinical trial for the low back, we’ll be headed to the knee next.”
References
- World Health Organization. (Jun. 19, 2023). Low Back Pain. Accessed: Aug. 9, 2023. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/low-back-pain
- D. P. Beall et al., “Abstract 45: Treatment of painful lumbar degenerative disc disease: A feasibility study,” J. Vascular Interventional Radiol., vol. 33, no. 6, pp. S21–S22, Jun. 2022.
- Internal data on file at ReGelTec, Baltimore, MD, USA.
- T. R. Oegema Jr. et al., “Comparison of the biochemistry of proteoglycans isolated from normal, idiopathic, scoliotic and cerebral palsy spines,” Spine, vol. 8, no. 4, pp. 378–384, May/Jun. 1983.
- A. Matta et al., “NTG-101: A novel molecular therapy that halts the progression of degenerative disc disease,” Sci. Rep., vol. 8, Nov. 2018, Art. no. 16809, doi: 10.1038/s41598-018-35011-4.
- A. Matta et al., “A single injection of NTG-101 reduces the expression of pain-related neurotrophins in a canine model of degenerative disc disease,” Int. J. Mol. Sci., vol. 23, no. 10, p. 5717, May 2022, doi: 10.3390/ijms23105717.
- L. I. Silverman et al., “Perspectives on the treatment of lumbar disc degeneration: The value proposition for a cell-based therapy, immunomodulatory properties of discogenic cells and the associated clinical evaluation strategy,” Frontiers Surgery, vol. 7, Dec. 2020, Art. no. 554382, doi: 10.3389/fsurg.2020.554382.
- DiscGenics. (Jan. 23, 2023). DiscGenics Announces Positive Two-Year Clinical Data from Study of Discogenic Progenitor Cell Therapy for Degenerative Disc Disease. Press release. Accessed: Aug. 17, 2023. [Online]. Available: https://www.discgenics.com/news-posts/2023/1/23/discgenics-announces-positive-two-year-clinical-data-from-study-of-discogenic-progenitor-cell-therapy-for-degenerative-disc-disease