“More than 600,000 people will die from cancer in the United States this year, and almost all of them could have been saved by detecting their cancers earlier when they were more amenable to the treatments available today,” according to Isaac Kinde (Figure 1), M.D., Ph.D., head of research and innovation and a co-founder of the cancer-screening company Thrive, headquartered in Cambridge, MA [1].
The earlier a cancer is caught, the easier it is to treat. With that as their mantra, numerous companies and research groups are working on blood-screening tests that can help catch cancer in its nascent stages. Approaches are wide-ranging and include such things as a simple blood draw to pick up genetic and immune-cell abnormalities, a subcutaneous cancer trap that can be periodically biopsied, and a portable device that can screen large volumes of blood. The methods may vary, but all are designed to spot cancer long before any symptoms appear.
Simple blood draw
At Thrive, which launched in April 2019 with US$110 million in venture capital funding, the company is developing and commercializing a liquid-biopsy test based upon technology developed by researchers at Johns Hopkins University. Within just six months, the company’s staff had grown from 25 to 50 and added a Cambridge, MA, location.
Called CancerSEEK, the test scrutinizes a simple blood draw not for the cancer cells themselves, but for cancer’s calling cards, specifically genetic mutations in 61 regions of 16 genes, as well as eight additional protein biomarkers that serve to identify the early stages of eight common cancers [2].
Dr. Kinde explained, “Cancers at the earliest stages can shed minute amounts of material into the plasma, so it’s very important to have a highly accurate method to interrogate the DNA bases that we isolate, and to do that, we use a combination of high-throughput sequencing technology, as well as an error-correction technology, which was also developed at Johns Hopkins, to accurately identify rare variants” (Figure 2). In addition, CancerSEEK analyzes white blood cells as a way to identify those mutations that are actually arising from benign neoplasms rather than cancer. “This combination of wet-lab and bioinformatic techniques maximizes accuracy in DNA sequencing,” he said.
Thrive is currently finishing a 10,000-patient study of CancerSEEK, and the company hopes to use the results to help inform the design of future studies that it will use to pursue U.S. Food and Drug Administration (FDA) approval for the test, according to Kinde.

At the same time, Thrive is developing what it calls an “integrated service model” both to help clinicians interpret the test’s results to drive diagnostic follow-up, and to educate patients about the role of the test within their overall screening and diagnostic strategy.
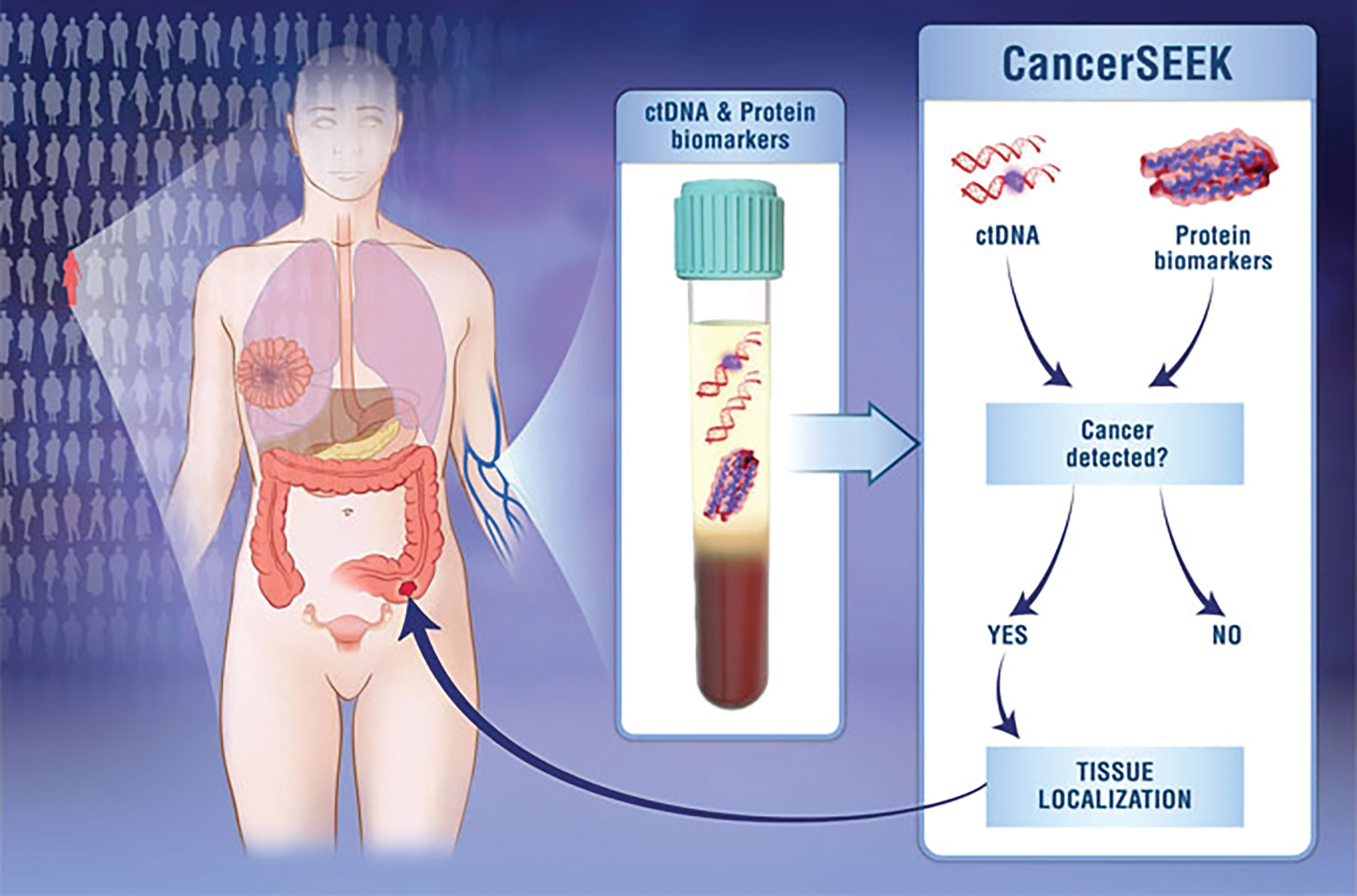
Details at https://hub.jhu.edu/2018/01/19/cancer-blood-test-johns-hopkins/.) (Image credit: Elizabeth Cook and Kaitlin Lindsay.)
While the 2018 study focused on eight cancer types, Kinde sees CancerSEEK as a multicancer test that could theoretically detect any type of cancer that sheds biomarkers into the circulating blood. With that capability and the clinical straightforwardness of the test, he envisions it becoming part of routine medical care. “You’d go to your doctor for a checkup, he or she would order the test, you’d have additional blood tubes drawn, the blood would get sent to us for analysis, and we’d send back the result along with supporting information as part of this integrated service model,” he described. “Keeping the process as simple as possible for the patient and the physician is key.”
Cancer-catching scaffold
Two research groups at the University of Michigan (U-M) are also developing cancer screening technologies, but in the form of two very different devices to catch tumor cells as they travel through the bloodstream.
One of the devices is an implanted scaffold that serves as a metastatic-site decoy for breast cancer cells that would otherwise spread into their favorite solid-organ targets, namely lung, liver, bone, and brain, according to Lonnie Shea, Ph.D., U-M William and Valerie Hall Chair of biomedical engineering. Shea’s counterpart in the research is Jacqueline Jeruss, M.D., Ph.D., who is a U-M associate professor of surgery and associate professor of biomedical engineering (Figure 3).
Their idea was to lure circulating tumor cells (CTCs) to the implant, so they could identify metastatic cancer earlier, slow cancer progression, and give interventional treatments a better chance of success.
The porous scaffold, which resembles a kitchen sponge measuring 5 mm in diameter and 2 mm thick, is inserted subcutaneously. Cells from neighboring tissue grow into the pores of the disc, and blood vessels also infiltrate to support the cells, Shea described. Concurrently, the immune system recognizes the scaffold as a foreign body and dispatches immune cells that travel to the scaffold. In the presence of cancer, another type of immune cell also rushes to the site. Known as myeloid-derived suppressor cells (MDSCs), these cells quash the immune response locally, and therefore provide a welcoming spot for cancer cells to engraft and multiply. The overall effect makes the scaffold an irresistible stop for CTCs, and delays their arrival into their typical solid-organ targets (Figure 4).
The researchers’ recent work demonstrated that they can detect cancer progression by looking for the genetic signature of immune cells, such as MDSCs, that recruit the cancer cells.
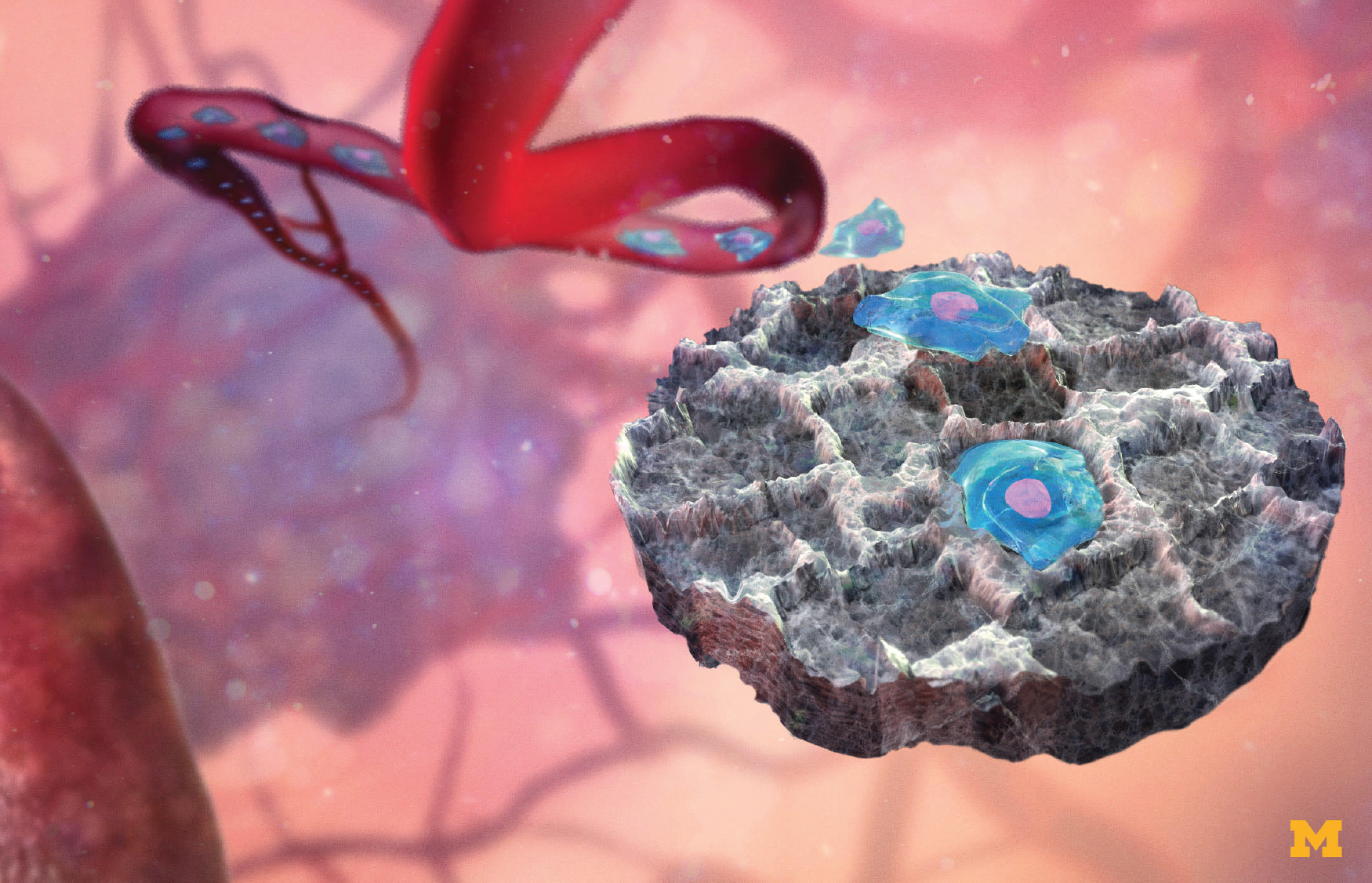
At that point, it is a simple matter to perform a core-needle biopsy of the scaffold and look for genetic signatures of MDSCs. “So by analyzing the immune cells, we can identify whether the scaffold contains healthy tissue or premetastatic tissue,” Shea said. “This provides potential platform technology so you can begin to analyze the environment in the implant as a way to identify a premetastatic stage, which would allow you to start to treat patients before the cancer begins to spread.” One clinical application, he noted, would be patients at high risk for distant recurrence, in which a patient is initially treated for disease at one site, but it returns elsewhere.
Currently, Shea and Jeruss are in discussions with the FDA to obtain clearance to begin a clinical study. As that progresses, they have begun expanding the project to consider noninvasive monitoring of the scaffold, possibly through the use of high-frequency spectral ultrasound imaging [5] or sensors incorporated into the implant, and are also beginning to investigate its potential use in screening for pancreatic, prostate, and ovarian cancer. Of the latter, Shea said, “It’s early, but I’m optimistic.”
External device that goes big
Another group at U-M is taking a different tack and is building a wearable device designed to screen a large volume of the patient’s blood [6].

“What really motivated this project is that we know the CTCs are the seeds of metastases and we find them even in the patients with early stages of cancer, so we didn’t want to miss any of these important cells,” said lead researcher Sunitha Nagrath, Ph.D., associate professor of chemical engineering and part of the U-M Rogel Cancer Center (Figure 5).
For this project, the researchers developed a system that continuously routes blood out of a vein via catheter, sends it into a small screening device that collects any CTCs, and then directs the CTC-free blood through another catheter and back into the vein. In designing the system, they had to move the blood quickly and smoothly through the device, so they put their expertise in microfluidic systems to work and devised a blood pump to maintain a sufficient flow rate and a separate pump to continuously infuse clot-preventing heparin. They then added a highly sensitive CTC-capturing chip, which she and other U-M researchers had developed previously [7], that uses antibodies to filter out the CTCs.
Testing of the device included injecting dogs with human CTCs (the cells cause no lasting harm to the dogs) and hooking them up to the system for a two-hour period. The test showed that the system not only worked well to move blood from vein to device and back to vein, but also captured 3.5 times as many CTCs as would be discovered through traditional methods that rely on a single blood draw. “We developed this technology as a proof of concept, so we only monitored one to two percent of the whole blood volume in the canine model,” Nagrath said, noting that her group is expanding the system capability to monitor a patient’s entire blood volume in as little as three hours, and hopes to begin pilot human trials soon.
“The immediate application we are working toward is to detect early recurrence (in cancer survivors), so that we can tell which patients need treatment,” she said. CTCs collected by the chips could also be used for downstream genotyping, or could be grown outside of the body and be used to assess which treatment options work best. “In other words, the patient could really receive personalized therapy because you could screen for potential therapeutics using the patient’s own cells,” she asserted. Beyond that, it might be possible to screen the full volume of blood of cancer patients following tumor resection as a way to eliminate any remaining CTCs before they leave the hospital, which she believes would greatly reduce the possibility of recurrence.
While the cancer work proceeds, Nagrath’s group is interested in using the device with other chips that can monitor for additional blood-borne entities, such as viruses, bacteria, or immune cells. Toward that end, they designed the system to be modular and showed that the chip can indeed be swapped out while in use. “The system has quite high sensitivity, so even if you have pretty infrequent cells that need to be detected in the blood, you could do it,” she remarked. With this in mind, the researchers soon hope to begin pursuing an FDA approval for the device as a way to look for not only CTCs but a wide range of blood-borne cells.
Early detection, early treatment
These three technologies use diverse approaches, but the researchers behind them all believe the same thing: it will soon be possible to identify various cancer types at ever-earlier stages when the disease is easier to treat.
Commenting on the scaffold approach, Shea remarked, “The idea that we can analyze the properties of this tissue to determine whether it’s normal, healthy tissue, or if it’s developing properties of a metastatic site—that’s what we hope to be able to discern. I think we have an opportunity to fill a void in what’s available for patients.”
Nagrath added that cancer-screening projects are moving forward rapidly today because of the contributions of many disciplines. Collaborators for the portable device her group is developing, for instance, represented such fields as medical oncology, clinical pathology, veterinary oncology, chemical engineering, and biomedical engineering. Working together not only brings varied expertise to the table, but also helps identify and solve challenges during technology development, she noted. “It’s a really exciting time, and as an engineer, I feel like every day there are so many opportunities for us to make a difference.”
A vast amount of academic and other research goes into new technological approaches too, noted Kinde. That includes CancerSEEK, which leveraged decades of cancer-genetics research to identify the disease mechanisms and “generate the type of evidence that’s needed to really make a big shift in how we identify cancers in patients.”
Kinde added, “For me, the problem is pretty well defined, and a solution is available, which is to find the cancers early enough where you can intervene and save people’s lives. Ultimately our mission is to save lives by making earlier cancer detection a part of routine medical care.”
References
- Thrive. Accessed: Dec. 10, 2019. [Online]. Available: https://thrivedetect.com/
- J. D. Cohen et al., “Detection and localization of surgically resectable cancers with a multi-analyte blood test,” Science, vol. 359, no. 6378, pp. 926–930, Feb. 2018.
- S. S. Rao et al., “Enhanced survival with implantable scaffolds that capture metastatic breast cancer cells in vivo,” Cancer Res., vol. 76, no. 18, pp. 5209–5218, Sep. 2016.
- R. S. Oakes et al., “Metastatic conditioning of myeloid cells at a subcutaneous synthetic niche reflects disease progression and predicts therapeutic outcomes,” Cancer Res., vol. 80, no. 3, pp. 602–612, 2020. doi: 10.1158/0008-5472.CAN-19-1932.
- G. G. Bushnell et al., “High frequency spectral ultrasound imaging to detect metastasis in implanted biomaterial scaffolds,” Ann. Biomed. Eng., vol. 48, pp. 477–489, Sep. 2019. [Online]. Available: https://link.springer.com/article/10.1007/s10439-019-02366-2, doi: 10.1007/s10439-019-02366-2.
- T. H. Kim et al., “A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells,” Nature Commun., vol. 10, Apr. 2019, Art. no. 1478. [Online]. Available: https://www.nature.com/articles/s41467-019-09439-9, doi: 10.1038/s41467-019-09439-9.
- H. J. Yoon et al., “Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets,” Nature Nanotechnol., vol. 8, no. 10, pp. 735–741, Oct. 2013.