One December day in 2013, Michael Rasmussen realized that just chewing his food made him tired. Short walks felt draining. At one point, he became so tired that he sat down and didn’t get up for three days. Rasmussen had been a man in perfect health. For 30 years, he lived as an artist in the Aleutian Islands in Alaska and walked or biked everywhere. He even bicycled across the United States five times. But now he had slid into a horrible medical mystery.
A chest cold became pneumonia. He had asthma attacks. He started bleeding out of his colon. He was scared. He was diagnosed with ulcerative colitis, but the treatment didn’t work. He was referred to a urologist, to a pulmonologist, and to a nephrologist. In the process, the uninsured Rasmussen racked up thousands of dollars in medical bills. And then, in the spring, a nephrologist ordered a kidney biopsy and stained it with Congo red, a dye that sticks to a substance called amyloid. Now, Michael finally had a diagnosis: amyloidosis. Although Rasmussen had hoped to be part of a clinical trial for a stem cell treatment, his disease had progressed too far. Instead, he entered hospice and died in October, just six months after his diagnosis [1]. (These details are from accounts provided by Michael and his sister Pamela that were previously featured on the Amyloidosis Foundation website [1]. On the website are many similar patient stories—stories that show how difficult it can be to diagnose and treat this rare disease.)
Amyloidosis occurs when copies of a misfolded protein called amyloid stick together to form amyloid fibrils that accumulate in major organs and tissues. Amyloid may build up in a single organ or in organs and tissues throughout the body. The involvement of multiple organs makes the disease difficult to diagnose because there are many possible symptoms—including abnormal heart rhythm, fatigue, numbness in hands and feet, swelling, weight loss, joint pain, problems swallowing, decreased urine, and skin changes—and many of these symptoms could just as easily indicate other diseases.

There are multiple forms of amyloidosis. Familial amyloidosis is hereditary and usually affects the nerves and kidneys. Secondary amyloidosis is caused by chronic infections such as tuberculosis, rheumatoid arthritis, and some forms of cancer. Primary amyloidosis is the most common form of the disease, but it is still rare. In the United States, around 3,000 people are diagnosed with primary amyloidosis each year. Because it can present in so many different ways, it often takes doctors a long time to make the diagnosis, and this delay can be very costly. Without intervention, the amyloid deposits can shut down major organs and kill the patient.
Even with a diagnosis, it is difficult to assess a patient’s prognosis or to know whether a given treatment—which can range from drugs to chemotherapy to stem cell transplants—is working. “Currently in the United States there is no way to do noninvasive imaging of systemic disease in these patients,” says Jonathan Wall, director of the Amyloidosis and Cancer Theranostics Program (ACTP) at the University of Tennessee Graduate School of Medicine (Figure 1, right – Photo courtesy of Kandi Hodges, University of Tennessee Graduate School of Medicine.).
Roadblocks to Imaging
Meanwhile, doctors in Europe have had access to imaging technology designed to view amyloid deposits for more than two decades. “In Europe you can get a whole body scan, and they can assess the amount of amyloid that you have in every organ,” says Wall. The imaging technique used in the United Kingdom involves isolating a protein called serum amyloid P (SAP) from donated blood. The protein is then purified, tagged with radioactive iodine, and injected into patients with amyloidosis. SAP binds to the amyloid, and six to 24 hours later the patient enters a whole body gamma camera scanner. The resulting image shows the location and severity of the amyloid deposits. “It’s a relatively low-resolution imaging technique, but in the U.S. we’ve got nothing like that,” says Wall. In the past 25 years, the National Amyloidosis Centre in the United Kingdom has performed 10,000 SAP scans [2].
It may seem bizarre that patients in the United Kingdom have had access to an imaging technique that is not available in the United States, but in the United States, the Food and Drug Administration requires that donated blood undergo stringent antiviral purification steps before products from the blood can be injected into another person. This protects patients from potential viral infections. Unfortunately, these purification steps also destroy the SAP protein.
This lack of imaging capability is a problem for physicians trying to treat amyloidosis patients in the United States. Even in patients who clearly have amyloidosis, it can be hard to know the extent of the disease and which organs are involved. “Amyloid is a very clinically challenging disease,” says David Aljadir, a hematologist and oncologist also at the University of Tennessee Medical Center. “We don’t have an available modality that can reliably detect amyloid deposits, especially in areas that are otherwise difficult to biopsy—such as the heart, kidneys, or nerve tissue.”
Clinicians also have difficulty seeing how amyloidosis patients are responding to treatment, information that is crucial given that many of the patients only have nine months to three years to live. “These patients also have a relatively poor prognosis—a lot of them—so they have a short life span once they’re diagnosed,” says Wall. “If you take 12 months providing a treatment that’s not working, but you can’t tell, then that’s a lot of time.”
A synthetic peptide Offers an imaging solution
When Wall came to the United States after completing his Ph.D. degree in the United Kingdom, he was committed to finding an imaging solution for amyloidosis patients and their doctors. Wall and his team knew that amyloid deposits are full of protein fibrils as well as sugar molecules called heparin sulfate proteoglycans. These sugar molecules are normally produced by every cell in the body, but the form that binds to amyloid has a distinct structure and electrical charge. Wall wondered if he could take advantage of these properties to make a synthetic imaging molecule that would selectively bind to amyloid deposits.
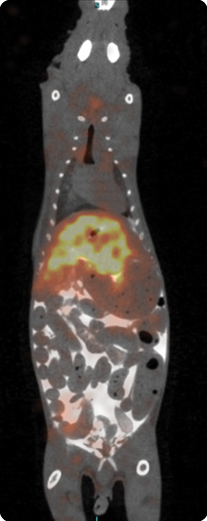
After producing a series of potential heparin-binding peptides, radiolabeling them, and injecting them into mice with amyloidosis, Wall’s team found a promising candidate, p5, which stuck preferentially to the amyloid deposits. The bodies of the mice cleared the unbound p5 quickly, which lessened their radiation dose—an important consideration for an imaging probe designed to one day be used in humans (Figure 2, right – Imaging amyloid in a mouse. (Image courtesy of the ACTP, University of Tennessee Medical Center)). The peptide can be labeled with several different radioactive markers and can be visualized with either positron emission tomography (PET) or single-photon emission computed tomography (SPECT) imaging [3].
As the group worked to further engineer their peptide, they found that adding a tail of amino acids to the peptide made it stick even better to the heparin (Figure 3). Wall says, “We’ve hit upon the peptide that we want to take into man.” They named the new peptide p5+14 (because it has 14 extra amino acids). The peptide’s stickiness is a prime advantage for getting “really informative high-resolution images,” according to Wall.
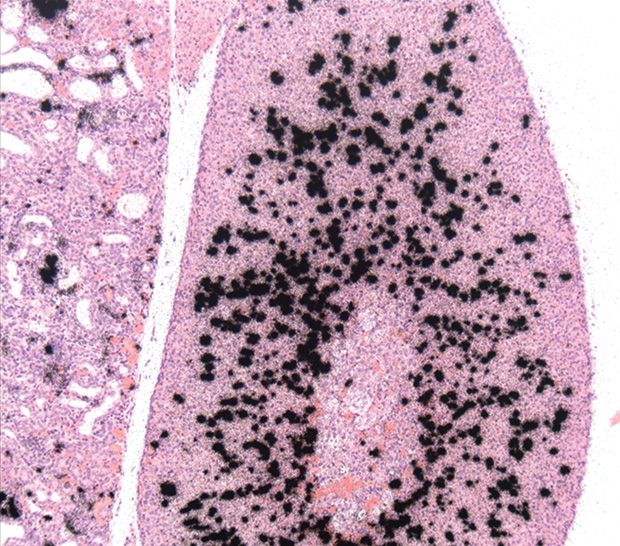
In fact, the resolution of the images means that using p5+14 could be more powerful than the technique used in the United Kingdom. Another advantage is that p5+14 doesn’t require blood donors or any biological materials (Figure 4). It also may make treating amyloidosis patients quicker and cheaper because clinicians will be able to tell if a particular treatment is working faster. “If you could image them quickly and see if the amyloid is being removed in response to therapy, then you can either stay the course on that drug or, if it’s not working, you can move on to something else,” says Wall.
Moving from Mouse to Man
Of course, none of this will be useful if p5+14 isn’t validated in humans. Wall and his team say they’ve done extensive preclinical validation in mice and are eager to bring the technique into human clinical trials (Figure 5). However, since it is a diagnostic agent (rather than a therapeutic agent) for a rare disease, p5+14 is a bit of tough sell for industry.
Still, Wall has kept at it. He applied to be a part of the National Heart, Lung, and Blood Institute’s Science Moving Towards Research Translation and Therapy program [4], which “provides tailored pharmacology and toxicology testing, manufacturing services, and regulatory support to investigators to expedite the transition of their discoveries to the clinic.” So far, this program has agreed to support Wall through the regulatory process and to provide enough human grade peptide to be used in 500–600 patients during a future Phase I clinical trial.

If p5+14 is validated in human patients, Aljadir says clinicians would “absolutely” find it useful. “Imaging studies like this may do for amyloid what PET scans have done for other solid tumor cancers—provide diagnostically sensitive and specific information that may be used to establish diagnosis as well as monitor response to therapy.”
While this type of peptide imaging could help doctors monitor patients’ responses to different treatments, it’s an open question whether imaging with p5+14 would replace Congo red in diagnosing patients. Using the Congo stain on a biopsy costs around US$50, whereas PET or SPECT imaging with p5+14 would likely cost thousands of dollars. “That being said, an imaging modality that could identify potential targets for biopsy would be a major leap forward in this disease,” says Aljadir.
Beyond Rare Disease
There are still several hurdles to pass before Wall will know whether or not p5+14 will be validated for use in humans and raise the standard of care for amyloidosis patients, but he’s already set his sights on other potential uses for the peptide.
Cerebral amyloid angiopathy (CAA) is a major cause of stroke in older adults. In patients with the disease, amyloid deposits can cause cracking in blood vessels in the brain, leading to bleeding and eventually strokes. Imaging with p5+14 could hypothetically be used to monitor the amount of amyloid in the brains of patients at risk of CAA before they have their first stroke.“We have evidence in mice that our peptide likes the amyloid in the vasculature around the brain,” says Wall. “And we’re working on a way to image it.”
Type 2 diabetes is another potential target. Patients with the disease have accumulations of amyloid in their pancreas, although the clinical usefulness of visualizing that amyloid is unclear at this point.
But Wall is most interested in the so-called “theranostic” potential of p5+14 and related peptides. Theranostics are molecules that can be used both in diagnosing and treating a disease. Wall and his team have found that some of their peptides have “inherent therapeutic properties.” These peptides can actually stop amyloid deposits from growing. “The idea that you could use the same peptide to image and effect some kind of therapy is the path that we’re going down now in a fairly big way,“ says Wall.
Wall is particularly intrigued by the possibility of developing immunotherapeutics for amyloid diseases—peptides that would make amyloid a target for antibodies present in the body so the immune system destroys it. This type of therapy could have potential for amyloidosis, and new treatments for the disease are sorely needed—as of now, there is no cure for amyloidosis.
References
- Amyloidosis Foundation. [Online].
- UK National Amyloidosis Center. [Online].
- J. S. Wall, E. B. Martin, T. Richey, A. C. Stuckey, S. Macy, C. Wooliver, A. Williams, J. S. Foster, P. McWilliams-Koeppen, E. Uberbacher, X. Cheng, and S. J. Kennel, “Preclinical validation of the heparin-reactive peptide p5+14 as a molecular imaging agent for visceral amyloidosis,” Molecules, vol. 20, no. 5, pp. 7657–7682, 2015.
- NHLBI Science Moving Towards Research Translation and Therapy Program. [Online].