Although the overall incidence of colon cancer has been falling over the past few decades, a pair of recent studies revealed a startling trend. In February 2017, researchers published a report showing that colon cancer rates were rising among younger adults. Some skeptics suggested the spike might simply reflect earlier detection and not necessarily represent a real increase in the disease. But a follow-up study found that Gen-Xers and millenials aren’t just getting cancer diagnoses earlier; they are dying from colon cancer at slightly higher rates than in previous decades. Death rates among adults aged 20–54 have increased slightly, to 4.3 deaths per 100,000 people in 2014, up from 3.9 per 100,000 in 2004.

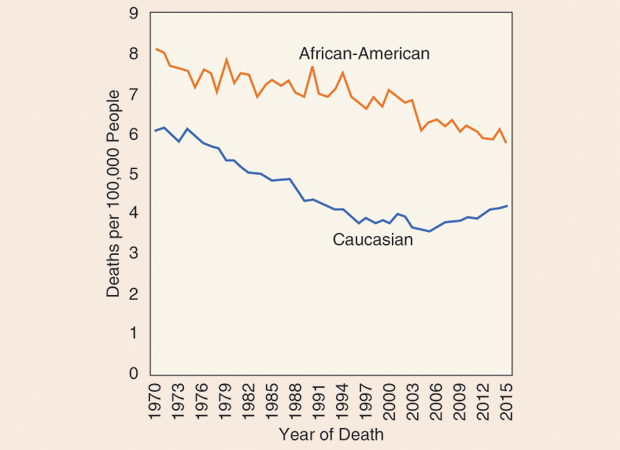
“The fact that death rates are increasing confirms or gives credence to the fact that there is a real increase in disease occurrence,” says Rebecca Siegel, an epidemiologist with the American Cancer Society and the lead author of the two studies (Figure 1, right). “Although there are some pretty well-established risk factors for colon cancer, like excess body weight and physical inactivity, we do not completely understand what is behind the rise among younger people,” Siegel continues. Interestingly, the increase in death rates appears to be confined to persons of primarily Caucasian ethnicity, while the death rates for African-Americans have not increased (Figure 2).
Colorectal cancer is the second-leading cause of cancer deaths, claiming the lives of about 50,000 Americans each year. Researchers are addressing colon cancer on two main fronts: detecting the disease earlier and treating it more effectively should it occur.
Finding Cancer Early Is Key
Due to its slow progression and the much-improved prognosis of patients diagnosed at early stages, screening for colon cancer is critical. Current guidelines call for regular screening for colorectal cancer to begin at age 50, and there are a number of screening tests available. The gold standard is the colonoscopy, an examination of the lining of the colon and the rectum using a fiber-optic camera. Colonoscopies provide both high sensitivity and specificity and can detect and remove precancerous polyps. However, the process is expensive, time consuming, and unpleasant. Stool tests are a noninvasive option used to detect abnormal blood or DNA markers that indicate malignancies. Although these tests are easier to use, they are not as sensitive as invasive screening methods. “There are lots of options,” says Siegel. “Not everyone wants to have a colonoscopy, and that’s okay, because the best test is the one that gets done.”
Even as public health experts like Siegel encourage more Americans to get screened by current methods, the search continues for better ways to screen for colon cancer. In 2016, the U.S. Food and Drug Administration (FDA) approved the first blood-based test for colon cancer. The Epi proColon test uses a polymerase chain reaction to detect the methylated Septin 9 gene that is present at greater levels in patients with colon cancer. Unfortunately, this test is neither as sensitive nor as accurate as either colonoscopies or stool-based tests. Some studies suggest the blood test may not be able to reliably detect cancer early enough for successful treatment, but there is evidence that some people who are reluctant to undergo the usual screening procedures would be receptive to a blood test. Approximately one in three Americans who should be screened for colon cancer are not; offering a blood-based test option might increase screening participation and adherence rates.
Despite the current limitations, blood-based screening tests may have a future. Epigenomics, the company behind the Epi proColon, recently announced a postapproval study in collaboration with the FDA, evaluating the test’s participation and performance among 4,500 individuals for the next three years.
Other researchers are looking to technology that can’t be seen with the naked eye to spot cancerous or precancerous cells through nanotechnology. Nanoparticles for medical use are usually no larger than about 100 nanometers (for comparison, a human hair is about 100,000 nanometers in diameter). These particles can seek out and manipulate individual cells well before a visible growth has formed, making them a potential future option for detecting colon cancer even earlier than is currently possible.
The Center for Cancer Nanotechnology Excellence at Stanford University in California, part of the National Cancer Institute’s Alliance for Nanotechnology in Cancer, has developed a technique that uses gold nanoparticles to locate and bind to cancer cells. The gold nanoparticles are delivered into the colon with a standard endoscope. There, they light up only when bound to cancer cells. As light from a special endoscope is shined on them, the nanoparticles illuminate any cancer cells, making them stand out for easier removal. The team hopes to begin a clinical trial to test the safety of this approach in human patients by early 2018. “It really comes down to early detection,” says Siegel. “Colon cancer is usually a very treatable cancer when it’s diagnosed early.”
Toward More Personalized Treatments
Currently, there are many treatments for colon cancer depending on its stage, including surgery, radiation, and chemotherapy. “Although chemotherapy extends survival times and improves quality of life, it is by no means curative,” explains Michael Pishvaian, a gastrointestinal oncologist and assistant professor at Georgetown University’s Lombardi Comprehensive Cancer Center in Washington, D.C. “More needs to be done to get at the root causes of colon cancer to catch it and treat it earlier.”
One approach that has emerged recently is the use of genomic profiling to identify specific molecular changes in a person’s cancer cells. A patient’s cancer cells can be genetically analyzed to help predict outcomes and select medications tailored to his or her cancer subtype.
As researchers have learned more about the genetic and molecular changes in cells associated with cancer, they have developed new drugs to target those changes specifically. Unlike chemotherapy, which kills all fast-growing cells, these targeted therapies specifically seek out cancer cells, destroying them or preventing them from dividing. “The benefits of targeted therapies are moving forward hand in hand with molecular profiling efforts,” says Pishvaian. “We have the ability to dissect a patient’s tumor at the molecular level and look at a broad swath of gene mutations that may be relevant to the patient’s cancer.”
Several targeted therapy drugs are currently used to treat colon cancer, but researchers are continuing to determine the best way to administer these drugs and in what combinations with other therapies. Among the oldest targeted therapies are vascular endothelial growth factor drugs (e.g., bevacizumab), which block the growth of new blood vessels to the tumor. Another class of targeted therapy drugs, epidermal growth factor receptor drugs (e.g., cetuximab), bind to a protein on cancer cells and stop their division and growth. The key to targeted therapies is determining which tumors might respond to one of the available therapies. Some colon cancers have mutations in the KRAS, NRAS, or BRAF genes, which are involved in cell signaling and division, that make certain targeted therapies ineffective. Doctors now commonly test every colon cancer patient’s tumor for these genetic mutations before treatment.

There are dozens of ongoing clinical trials of new targeted therapies, and researchers continue to assess the actions of targeted therapy drugs by themselves and in combination with standard therapy. “We’re seeing a shift in drug development away from developing drugs for specific cancers and toward developing drugs for a particular genetic abnormality,” according to John Marshall, chief of the Ruesch Center for the Cure of Gastrointestinal Cancers at Georgetown’s Lombardi Comprehensive Cancer Center (Figure 3, right). Marshall says there have not been very many advances in colon cancer treatment since the early 2000s, when targeted agents like bevacizumab and cetuximab were introduced. However, immunotherapy—a treatment that uses the body’s own immune system to fight cancer cells—is a promising new avenue of therapy.
Pembrolizumab (Keytruda) is an immunotherapy drug that targets PD-1, a protein on immune cells. By blocking PD-1, the drug boosts the immune response against cancer cells. When Keytruda was first tested in patients with advanced colon cancer, the results were disappointing: only one out of 33 patients responded well to the drug. It turned out that the one patient who responded had a condition called mismatch repair (MMR) deficiency, which renders tumors incapable of repairing damage to their DNA. Such cancers are said to show high microsatellite instability (MSI-high). In a follow-up study, Keytruda brought the cancer under control in 90% of these specific patients. Unfortunately, only a minority of patients, somewhere between 3–5%, have MMR-deficient, MSI-high colon cancer, and these are the patients most likely to benefit from current immunotherapy drugs.
“Getting immunotherapy to work for more colon cancer patients would be the Holy Grail,” says Pishvaian. “There are dozens of trials out there trying different immunotherapy combinations and combinations with standard therapies in attempts to finally make immunotherapy work for the average colon cancer patient.”
While researchers continue to explore new ways to treat colon cancer, there is wide agreement that screening and early detection are essential. “Early-stage colon cancer has a high survival rate—a five-year survival rate of about 90%,” Siegel remarks. “We need to do our best to prevent disease by maintaining a healthy weight, leading a physically active lifestyle, and reducing processed meat intake. But when there is disease, we need to recognize the symptoms and have regular screening so the cancer can be diagnosed in its early stages and treated most effectively.”
Other Resources
- American Cancer Society. (2017, May). What’s new in colorectal cancer research? American Cancer Society. [Online].
- K. C. Birendra, J. J. Hwang, C. J. Farhangfar, and S. J. Chai, “Advances in immunotherapy in the treatment of colorectal cancer,” Amer. J. Hematology Oncology, vol. 13, no. 7, pp. 4–8, 2017.
- P. M. Boland and W. W. Ma, “Immunotherapy for colorectal cancer,” Cancers, vol. 9, no. 5, p. 50, 2017.
- S. Hagan, M. C. M. Orr, and B. Doyle, “Targeted therapies in colorectal cancer—An integrative view by PPPM,” EPMA J., vol. 4, no. 1, p. 3, 2013.
- I. A. Issa and M. Noureddine, “Colorectal cancer screening: An updated review of the available options,” World J. Gastroenterology, vol. 23, no. 28, pp. 5086–5096, 2017.
- National Institutes of Health. (2015, Aug.) Advances in colorectal cancer research. National Institutes of Health. [Online].
- B. Viswanath, S. Kim, and K. Lee, “Recent insights into nanotechnology development for detection and treatment of colorectal cancer,” Int. J. Nanomedicine, vol. 11, pp. 2491–2504, June 2016.