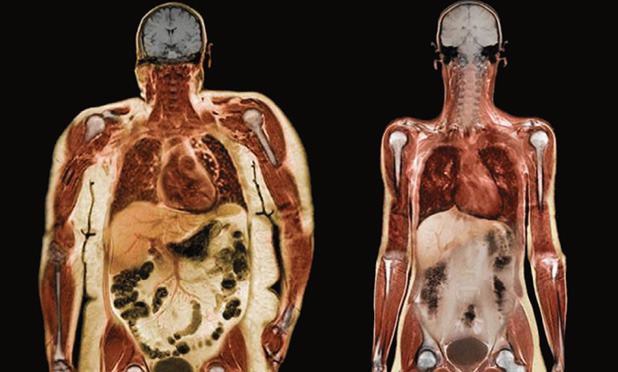
When asked about our weight, most of us can name a figure based on prior knowledge. And while stepping on a scale gives us the ability to know that exact number and track it routinely, it does not provide insights into our body’s composition. This, at the basic level, refers to proportions of fat and lean or fat-free mass (FFM) that comprise the human body. Conventionally, the body mass index (BMI), which is the ratio of body weight in kilograms to the square of its height in meters, and anthropometric parameters like waist circumference, waist-to-hip ratio, and skinfold thickness have been used to estimate the level of fatness. In fact, BMI is the de facto marker for stratifying individuals into underweight (<18.5 kg/m²), normal (18.5–24.9 kg/m²), overweight (25–29.9 kg/m²), and obese (>30 kg/m²) categories. Nonetheless, these metrics are limited in precisely characterizing individuals by percentages of body fat and muscle mass, particularly in epidemiological studies where these proportions vary across age, sex, and ethnic groups. Of note is also how, solely on the basis of BMI, a physically fit individual may be classified as overweight due to having a higher proportion of lean body mass, which outweighs fat. This highlights the importance of body composition in weight tracking and management.
Body-weight management is a global societal fixation in part due to the accelerating obesity pandemic and awareness that slimness is linked to well-being and success. Globally, the weight-management industry, heavily based on meal-replacement products and weight-loss supplements, is presently valued at US$14 billion, with an anticipated 3% compound annual growth rate through 2018. With a third of the sales, the United States commands a majority of this market. In the United States, the National Health and Nutritional Examination Survey (NHANES) estimates that the age-adjusted prevalence of obesity is 33% in men and 36% in women. Worldwide, recent figures from developed countries denote that the prevalence of adults with a BMI higher than 25 is 37% in men and 38% in women. Being overweight or obese is associated with functional limitations and occupational issues such as reduced spine flexibility, limited range of motion of the major joints, decline in endurance and muscle strength, and impaired respiration. Furthermore, excessive body fat predisposes individuals to chronic disorders such as hypertension, high blood cholesterol, diabetes, sleep apnea, coronary heart disease, stroke, and cancer.
The Skinny on Body Composition
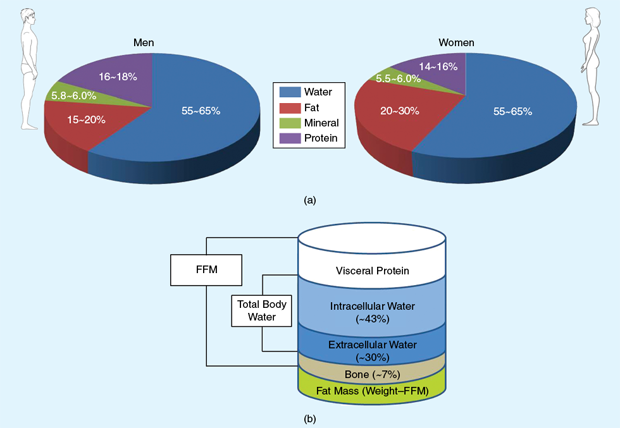
Our bodies are composed of water, proteins, minerals, and fat. Total body water is separated into water that resides inside (intracellular) and outside (extracellular) of cells. A simpler bicomponent model divides the body into adipose tissue, commonly referred to as (storage) fat, and lean tissues comprising muscles, bones, ligaments, tendons, and internal organs (Figure 1). A secondary category of fats, essential fat, is found in the marrow of bones, in the heart, lungs, spleen, liver, kidneys, intestines, muscles, and the central nervous system. Essential fat is critical for normal physiological functions including child-bearing and hormone-related processes and varies from 8 to 12% in females and 3 to 5% in males. The evaluation of body composition is vital in weight-management interventions where the aim is to bring down the proportion of adipose tissue or fat, which, if left unchecked, can lead to being overweight and, eventually, obese.
Research in human body composition began more than 70 years ago in the lab of Albert Behnke at the Naval Medical Research Center. Several methods were subsequently developed and introduced. The most common clinical techniques include hydrostatic or underwater weighing, air-displacement plethysmography (Bod Pod), dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA). Of these methods, DXA, which remains the gold standard for body-composition analysis, estimates total and regional body-fat percentage by utilizing a tissue specific model (fat, lean tissue, and bone) with the attenuation of low-dose X-rays dependent on thickness, density, and chemical structure of tissues under examination. BIA is the collective term applied to noninvasive methods that measure the body’s electrical response to the introduction of a low-level alternating current (ac) and the biophysical models to estimate body composition from such electrical measurements. The application of the measurement of impedance of the human body for estimating total body water was first published over 50 years ago by Thomasett. However, it was not until the early 1980s that impedance techniques were routinely used and clinically validated for evaluating fat and FFM of the human body.
In BIA, high-frequency ac (usually at 50 kHz and between 300 and 1,000 mA) is applied to the tissue through a set of drive electrodes. This ac results in a potential voltage change between a set of receptive electrodes. This voltage difference is related to the resistivity of the tissue between the voltage-sensing electrodes and the current that flows across the tissue. In essence, BIA measures the resistance of body tissues to the flow of a small, harmless electrical signal. The well-hydrated FFM (e.g., muscle) is a good electrical conducting medium, whereas the poorly hydrated adipose tissue (or fat) is a good electrical insulator. Impedance measurements and subject information such as height, weight, and gender are then used to predict body fat within an individual. The measured impedance varies according to the passage of current through fat and other tissues. Unlike DXA and underwater weighing, BIA is practical and does not have weight and height restrictions that can limit its use within certain populations.
As a consumer electronics company, Samsung’s interest in BIA devices, particularly as a health care tool, stems from the increasing attention that wearable/mobile health monitoring technology has received lately. The emerging patient-centric health care model has given consumers greater access to information, empowering them to make better personal health choices. Connected digital and mobile health will be vital moving forward in people’s lives as it offers wellness, fitness, adherence, and prevention, in addition to the routine play- and work-oriented features. Recent technological advances in wearable sensing, low-power integrated circuit design, and wireless communications enable concurrent monitoring of physiological signals such as heart rate, electrocardiogram, blood pressure, electroencephalogram, and blood glucose. With the increased awareness of the obesity epidemic and a collective focus on wellness and prevention, a pocket-sized unobtrusive BIA device could seamlessly be incorporated into an individual’s lifestyle, enabling self-quantification and providing sensory and visual feedback for enhanced weight management. Furthermore, Samsung has an established smartphone app, the U.S. Food and Drug Administration-approved S Health, which is rapidly shifting from a consumer fitness application into the chronic disease and medical domain. S Health can also serve as a gateway for BIA devices to share data with health care providers and other stakeholders to test and validate solutions for improving health outcomes through patient/consumer engagement.
The Nuts and Bolts of BIA
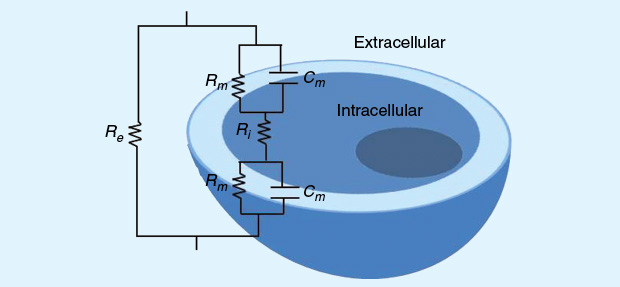
To understand BIA and the physical basis for extracting physiological parameters from the conversion of body electrical measurements, one has to appreciate the equivalent circuit model proposed by Fricke and expanded by Cole. This model relates extra- and intracellular ionic fluids as parallel resistors and the cell membrane as a capacitor (Figure 2). The flow of an alternating current [I(t)] through two points of a body is equal to the ratio of the voltage drop [V(t)] to the electrical impedance between these points [I(t) = V(t)/Z], according to a generalization of Ohm’s law. The absolute impedance at a measurement point includes resistance R and reactance Xc and is described as the square root of the sum of squares of the two entities:
![]()
Living organisms are made up of conductive and nonconductive components with fluid volumes, tissue properties, and cell membranes as the main electrically corresponding constituents. By considering an organism to serve as a component in a safe and controlled electric circuit, the change in voltage after application of ac would then yield bioelectrical measurements that can be related to various structural and functional biological variables. Here, resistance is the opposition to the flow of ac through intra- and extracellular ionic solutions. Conductance is the inverse of resistance and reactance is the delay in the conduction of the applied current by cell membranes and tissue interfaces. Capacitance due to the membrane’s properties is a function of reactance and causes the current to lag behind the voltage, resulting in a phase shift that is quantified as the angular transformation of the ratio of reactance to resistance [1].
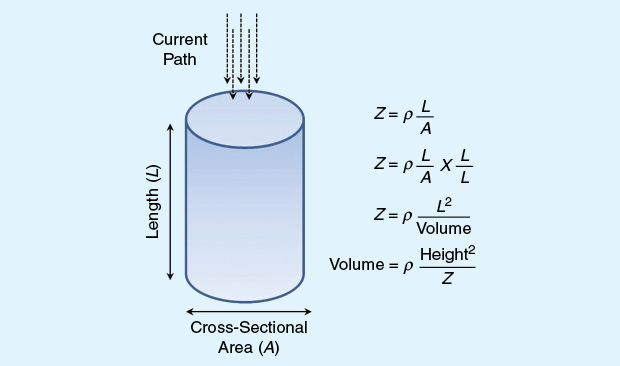
The physical model that relates impedance or resistance to volume is well-established. In a uniform conductor with constant geometry and composition, impedance is related directly to the product of specific resistivity and length of the conductor and indirectly to its cross-sectional area (Figure 3). Most BIA devices employ a single frequency at 50 kHz, operating under the assumption of a constant hydration of the fat-free body to estimate the FFM. However, at this single frequency, current flows primarily through extracellular fluids, affording narrow impedance spectrum information and potentially limiting the prediction accuracy of intracellular water fractions. For this reason, multifrequency BIA devices applying different frequencies (upward of four or more and ≥100 kHz) are preferred as current penetrates all body tissues, permitting an enhanced differentiation of total and extracellular fluid compartments in the body. This has further implications for improved predictions of the intracellular water and FFM due to reduced skin impedance and increased penetration of cell walls.
Various manufacturers have used hand-to-foot anatomical pathways, while some instruments use other pathways, such as hand to hand or foot to foot. Additionally, segmental BIA offers an alternate method for predicting body composition. It takes into account that the human body is complex in shape, varies from hand to foot or trunk, and is represented by five heterogeneous cylinders (e.g., arms, trunk, and legs), each with varying resistivity over which the resistances are measured separately.
The application of the conventional BIA model in biology hinges on some assumptions. The bioelectrical conductor is assumed to have a relatively homogeneous composition (i.e., constant composition and hydration) and constant geometry including cross-sectional area and shape. Based on this, the volume of a cylindrical homogeneous conductor is proportional to the square of its length (or height in humans) and inversely proportional to impedance, also known as the impedance index (height²/Z). Diverging from any of these assumptions affects the validity of this model in the assessment of health or disease. Further changes according to environment, temperature, and body heat or stress are not factored. Of course, the human body is not a homogeneous tube-shaped conductor, and the degree to alter the flow of electricity at a specific temperature is not constant. This alludes to the importance of developing revised estimation models factoring in weight, gender, and age. Further, extensive clinical experiments are needed to generate equations for body- fat estimations across both genders and several age groups to develop robust bodyimpedance analyzers.
Samsung’s R&D Efforts in the BIA Space
For the past few years, the research team within the Digital Media and Communication R&D center at Samsung Electronics (Suwon, South Korea) has been working on developing miniature multifrequency (pocket-sized) handheld BIA devices that can function as an accessory to one’s Samsung smartphone. In line with several parameters such as activity tracking, heart rate, sleep efficiency, and calorie consumption that can be monitored routinely with wearable devices such as the Galaxy Gear Fit or Gear S, Samsung’s goal was to also provide body composition assessments to consumers who have embraced the quantified-self (or personalized health) movement or who are interested in weight control and management.
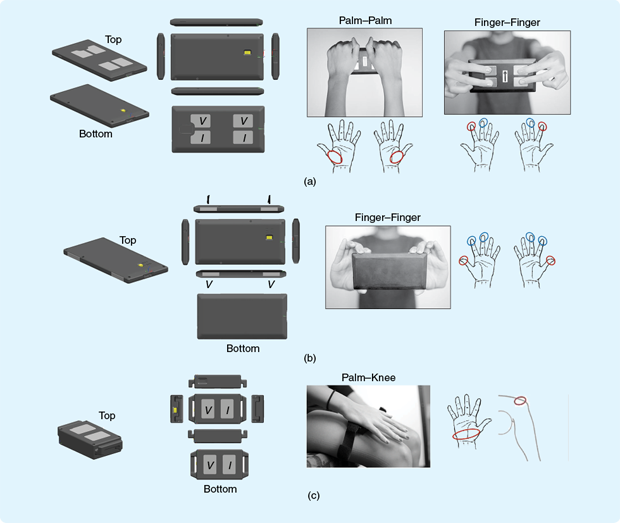
Three prototype BIA devices for measuring distinct anatomic pathways have been developed (Figure 4). We refer to the three devices as Samsung body-fat (SBF) meters. Each device measures impedance along a predesignated anatomical current pathway. For example, the SBF-01 device measures impedance along the palm-to-palm and one finger-to-finger pathway, where subjects maintain a standing posture with arms outstretched at 90°. Measurements with the SBF-11 device also utilize the aforementioned standing position for measuring impedance along an alternate finger- to-finger pathway. The third device, SBF-21, requires a seated posture for measurements made along the palm-to-knee pathway to closely model a segmental BIA system. Each SBF device comprises a system integrating an analog front end including four current-application and voltage-sensing electrodes in a tetrapolar configuration. Having four such electrodes helps eliminate the inaccuracies typically encountered with dual-electrode impedances. For each measurement, a sinusoidal current of <500 mA is applied. Furthermore, the devices include microprocessors and Bluetooth modules for connectivity and wireless data transfer and operate at five different frequencies (5, 50, 100, 150, and 200 kHz) for generating impedance data across the four anatomic pathways. The devices are rechargeable through a five-pin microuniversal serial bus (USB) port.
To effectively measure body fat and compartments such as muscle mass, these SBF BIA systems had to be calibrated against a reference gold-standard body composition method (e.g., DXA), a practice that is common among BIA device manufacturers. To garner interest in the SBF devices from both consumers and health care providers, the research team sought to undertake clinical studies for evaluating the performance of these systems and comparing them to gold-standard modalities. The underlying hypothesis was that development of BIA prediction equations for the Samsung devices across subjects could be accomplished by calibrating the BIA-derived data to body-composition data obtained from DXA. With BIA, the impedance output is related to the amount of conducting fluid, usually water and electrolytes. In turn, the amount of water present is related to the amount of body fat and muscle. Such relationships can then be incorporated into the device software by updating the existing algorithms.
As a first step toward achieving clinical validation of the SBF devices, a clinical trial involving an Asian population consisting of 256 adults spanning a wide range of ages and BMIs was undertaken in collaboration with researchers (Dr. Ji In Chung, Dr. Yoon Ju Na, and Dr. Jae Hwan Jee) from the Samsung Medical Center in Seoul, South Korea. The subjects were stratified into three BMI categories: underweight, normal–overweight, and obese. We first examined representative impedance values derived by the three SBF devices against corresponding electrical pathways and the whole-body electrical circuit measured using a clinical grade gel-electrode reference multifrequency BIA system from the InBody Company. Next, correlations between the impedance index derived from the SBF devices, InBody, and lean soft tissue as measured by DXA were evaluated. Such relationships provided general estimates of the performance of the SBF devices in developing body-composition-estimation equations.
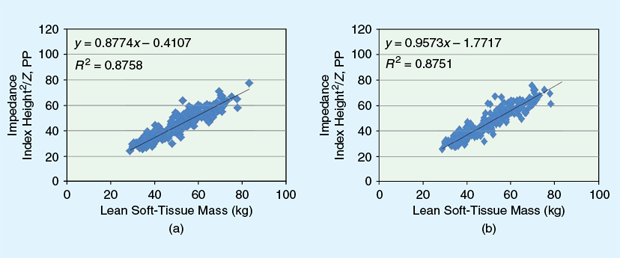
The first clinical study helped establish fat and FFM prediction equations for an Asian population. To advance the development of the SBF devices for characterizing body composition in other populations besides Asians, the R&D team at Samsung Research America (SRA) based in Richardson, Texas, undertook a second clinical validation trial by collaborating with Dr. Steven Heymsfield and Dr. Jolene Zheng of the Pennington Biomedical Research Center (PBRC), a Louisiana State University-affiliated institution in Baton Rouge. The PBRC enjoys an international reputation for its cutting-edge research in chronic diseases, nutrition, and body composition. A phase I study at the PBRC included 311 adults (primarily Caucasian and African American) using a testing and validation protocol that was identical to that of the Korean study [2]. The results showed strong correlations between the four anatomical pathways at 50 kHz and the corresponding InBody electrical pathways (R2, 0.83–0.96). Similar significant associations were seen between the impedance index from the SBF devices and the DXA-based lean soft-tissue regions (Figure 5). The SBF systems show promise as a means of quantifying an individual’s body composition using small-formfactor mobile devices for predicting body-fat percentage.
We are currently undertaking a phase II trial at PBRC on 100 children and adolescents between the ages of 10–17 with the SBF devices by following similar protocols as in phase I. The impetus for undertaking such a study in a much younger U.S. population relates to the 17% prevalence in obesity in this age group, according to NHANES. This figure is even higher worldwide (24%). Prevention of childhood obesity or efforts to engage younger generations in healthy weight management may lead to an increase in adoption of the SBF meters by younger age groups, prompting the need to develop the appropriate body-composition-prediction equations. In addition to this, recent work at SRA has involved the development of novel multifrequency BIA analog front-end integrated circuits featuring higher frequencies (up to 1 MHz) and operating at substantially lower power relative to the current system. These developments are aimed at facilitating segmental and whole BIA measurements on par with clinical-grade devices.
Technological advances and clinical validation have enabled the use of BIA for body-composition monitoring for over 30 years. However, the rise of digital and mobile health innovations allows Samsung to integrate BIA as an additional physiological sensing modality in small-form-factor mobile devices for seamless lifestyle incorporation and to achieve more user-centric health management. The BIA clinical trials are helping to validate the devices along with the body-composition- prediction equations and algorithms to increase awareness among both consumers and their health care providers.
References
- H. C. Lukaski, “Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research,” Eur. J. Clin. Nutrition, vol. 67, Suppl 1, pp. S2–S9, Jan. 2013.
- S. B. Heymsfield, J. Zheng, M. Wang, C. Gao, J. Y-H. Kim, A. Choi, S. W. Jo, J.-T. Oh, J. Cho, Y. A. Bhagat, and I. Kim, “Evaluation of novel hand-held wireless bioelectrical impedance analysis (BIA) body composition devices,” FASEB J., vol. 29, no. 1, p. 747.2, 2015.