The need for viable global health solutions—and particularly solutions for global surgery—are paramount [2]. In response, a research group at the University of Leeds, U.K., has been working in concert with a multidisciplinary team to develop retractor for abdominal insufflation-less surgery (RAIS), a gasless laparoscopy system that enables crucial surgery in low-resource settings. The group is led by Peter Culmer, professor in health care engineering at the University of Leeds (Figure 1). Recently, their paper, “The RAIS Device for Global Surgery: Using a Participatory Design Approach to Navigate the Translational Pathway,” published in the IEEE Journal of Translational Engineering in Health and Medicine [1], was selected to receive the 2023 IEEE Engineering in Medicine and Biology Paper Award.
IEEE Pulse had the opportunity to speak with Culmer about this novel project and the lessons learned in designing and manufacturing a surgical device for use globally.

Figure 1. Prof. Peter Culmer speaking about the RAIS device at ICIGS2023, Kenya 2023. (Photos courtesy of Peter Culmer.)
IEEE Pulse: Could you share how and why your team became involved in this global health initiative?
Culmer: Our work in this area was initiated by the Global Health Research Group, a project sponsored by our National Institute of Health Research. The remit of that group was to look at surgical provision across the world and identify unmet needs. There’s this rather stark statistic from World Health Organization studies that found over 5 billion people around the world have no access to surgery or safe surgery, and this lack of access skews much more toward people living in low-resource settings.
For this project, we looked at the provision of laparoscopic surgery, also known as keyhole surgery, to try to understand why there was this underserved provision. Our group was interested in exploring solutions here because the clinical specialists had identified this type of surgery as difficult to deliver in low-resource settings. It requires significant amounts of resources and trained people, which means there are real barriers to implementation.
On the other hand, this surgery is very appropriate in the sense that it allows faster recovery for the patient. That is, rather than an open surgery, which has a higher risk of infection and may take up to a week for recovery, with keyhole or laparoscopic surgery the patient might be out of the clinic the same day or within a day. For people who depend on work to support their family, laparoscopic surgery is brilliant because it enables them to get back into the working world much more quickly.
IEEE Pulse: What were some of the initial challenges?
Culmer: The overarching challenge was to bring the laparoscopic technique to those low-resource areas. We needed to identify how best to deliver the surgery as well as consider the barriers to implementation. To facilitate this, the Global Health Research Group identified people who were doing work in rural areas of India near the Himalayas—northeast India predominantly. They were using a technique called gasless laparoscopy, which is quite a unique approach because it gets around some of the key barriers to standard laparoscopic surgery.
For standard laparoscopic surgery, one of the vital resources you need is CO2 gas, and you need a regular supply. You also need general anesthesia. These drugs are fairly expensive, and they require a team to deliver and monitor them. Gasless surgery gets rid of the gas and uses a mechanical lift device. That is, rather than using gas to inflate the abdomen, which creates the space for the surgeon to work, instead you use a lift device that pulls the abdominal wall up to create that space and give the surgeon the room to operate.
The second thing is because you are using a lift, you can actually use a different form of anesthesia—spinal anesthesia. It’s cheaper and easier (than general anesthesia) because the surgeon can administer the spinal anesthesia themselves. It doesn’t require an anesthesiologist, which means your dependency on that larger group of trained people is lowered. In addition, this gasless approach is a great technique for a set of key procedures called “bellwether” procedures. These include the most common kinds of surgeries—the often life-saving procedures—including removing the gallbladder, removing the appendix, hysterectomies, and diagnostic laparoscopy.
The aspect we identified as engineers was the need for improved equipment. There was a lift device that existed—it worked but it didn’t really meet modern standards. The device was difficult to clean and sterilize, and it was heavy. That was our route into the project, where we thought here’s an opportunity and a need for a modern piece of surgical equipment that can meet the requirements of these surgeons. The goal was that hopefully by developing a more appropriate device, we could help lower the barriers to this surgery being used.
IEEE Pulse: Once you had identified a potential need, how did your team go about creating a viable product?
Culmer: The key was going through a process where we worked closely with a product design team—Pd-m International (https://www.pd-m.com/). The first step was understanding the needs of the stakeholders, principally the surgeons in this case. To accomplish this, we worked closely with the surgeons and nursing staff—watching them do some procedures and spending time with them in the operating theater with the existing equipment to understand how it works and what they would want from an improved system (Figure 2).

Figure 2. Dr. Jesudian Gnanaraj (front right) introduces our design team to gas-less laparoscopic surgery. (Photos courtesy of Peter Culmer.)
That interaction was really crucial, and it highlighted the fact that a low-cost system was an important factor. Maintenance was also important because often these surgeries take place in remote or rural settings where you don’t want to rely on consumables, but equally you don’t want to have to be repairing it all the time. It was clear the technology needed to be robust, but if you did need to repair it, it should be amenable to being repaired. Also, the device needed to be lightweight, as sometimes people would need to transport it, and the existing device had some really heavy components that made it difficult to carry around safely. Other important aspects for us were cleaning and sterilization. We wanted to design the system to modern standards, so it had to be compatible with an autoclave system. The existing system couldn’t really strip down. Instead, you had to wipe it with sterilization fluids. The new device is designed as a modular system where you can take it all apart, scrub each part, autoclave it, and then reassemble it.
Another key factor was usability. We wanted the system to fit in with the way the surgeons work. Sometimes they work with just the lead surgeon and maybe a single assistant or nurse, so they might not have a big team around them, and that means if they need to adjust the equipment, they need to be able to do it themselves while maintaining stability in a sterile environment. Making it easy for them to adjust was crucial.
Overall, we were keen to adopt a process officially called “participatory design” where the idea was that after we identified the needs, we worked closely with the surgeons, and then kept working with them all the way through the design process (Figure 3). In this way, we developed some initial concepts with their guidance, and then we would run through the concepts with them. Getting their feedback quickly was really helpful for us to move toward a more final prototype.

Figure 3.The RAIS device is iteratively developed in close collaboration with surgical stakeholders using participatory design principles. (Photos courtesy of Peter Culmer.)
One feature that took us a little while to understand was the best way to lift the abdominal wall. Essentially, there’s a little ring that you insert into the belly button, which looks somewhat like a corkscrew. If you make a small incision and insert the ring, you just lift up vertically and we thought that would be sufficient. That was our understanding from a naive point of view, and our initial concepts worked on that principle. However, we received feedback from the surgeons that actually what they did was angle the rings, essentially positioning and tilting them to provide the right angle to lift the abdominal wall in different directions and in different ways. It was quite nuanced and easy to miss because it wasn’t a huge tilt, but it was enough that if they tilted it in the direction that they were operating, it would give them a much better view and they could perform more efficiently (Figure 4). Without being able to do that, the system wasn’t meeting their needs. So those little fine bits of detail were really important.
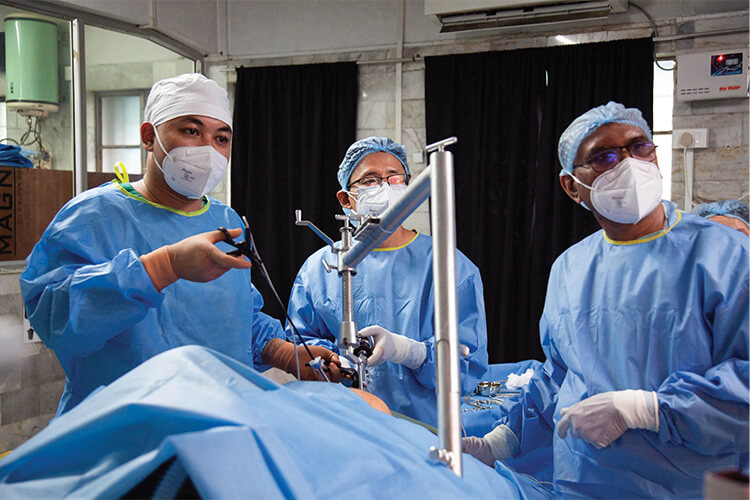
Figure 4. The RAIS system in use performing gas-less laparoscopic surgery. (Photos courtesy of Peter Culmer.)
IEEE Pulse: Were there any key lessons learned throughout the process of manufacturing the device?
Culmer: One thing is the need to work within the local context. For example, we developed a design at the University of Leeds and were able to fabricate it in our machine shops, but then we needed to get it made in India through our industry partner (a company called XLO—Ortho Life Systems). A lot of interaction is required in the process of deciphering how to translate your prototype design into a system that can be manufactured and, in this case, manufactured in India. So, if you’re designing equipment for a particular region, it’s interesting to see how you can have it produced locally and how you can work with local expertise.
In addition, we’re increasingly interested in working with academics and engineers in local regions. We have some links with Kenya and Uganda, both from a clinical point of view, but also from an engineering point of view (Figure 4). There is real enthusiasm in those regions to develop capacity to innovate and to produce innovations for health care. As seen during the pandemic this is crucial because supply chains can fail, so it’s important to have local capacity. And that’s fantastic because we would like to work in collaboration. We don’t want this to be something that’s developed in a high-income country, and then dropped into a different context. It should be a collaborative effort across all disciplines, and that knowledge exchange is really important. We’ve learned a lot from the engineers and engineering teams that we’ve met in Kenya and Uganda, so it is definitely a reciprocal arrangement. I think that’s actually one of the most valuable things that is often overlooked.
IEEE Pulse: What experiences did your students gain from the project?
Culmer: In the U.K. and at the University of Leeds in particular we’re encouraged to link our research to our teaching, and I always speak to something around global health in the projects that I offer to students. Engineers want to work in this area—they see the value and potential impact and they’re really driven. These projects also offer a good way of highlighting the need to be efficient and to adopt robust design processes, so inherently it comes back to a lot of fundamentals in terms of good design and good engineering.
I think one of the things they loved about this is that you’re designing for a low resource setting, so you need to keep things as low cost and simple as possible. It’s often called “frugal innovation” or “frugal engineering,” which presents an interesting set of engineering challenges that are nontrivial to solve. You’re designing products for challenging environments to a tight specification, so it underpins a lot of the things that we would teach anyway, but students really enjoy the added effect of the real-world impact of what they are working on.
IEEE Pulse: Congratulations on the paper award. What about this project do you feel had an impact on how the paper was received?
Culmer: The comments we’ve received reflect the fact that it differs from your standard engineering paper in that we talk a lot about the process of working together as a multidisciplinary team, which is often present in engineering but particularly so in this case. When working in health care, it’s necessary, but when working in health care for low-resource settings, it’s crucial. Working with the clinicians, the surgeons—the people who will use the equipment—right from the outset to understand their particular needs is really important.
In addition, a successful process is not just having a single point of contact with stakeholders but actually starting a continual series of meetings and exchange of information. All through the work we’ve done, we’ve had close contact with those clinicians and industry representatives as well. Our emphasis has been to try and get the technology out into the world and to have it used. To do that, to make it successful, you need to look at all those translational areas right from the outset and build that into the process.
IEEE Pulse: Do you have some particular advice that you have gained through this effort that others might use as a model for translation success?
Culmer: We were fortunate that we started the work and had some initial contacts prepandemic. In this way, we were able to establish some close working relationships through in-person meetings and then we traveled to India, which was really instrumental in building relationships. We were able then to sustain the interaction with calls, and obviously the pandemic necessitated that, but actually due to the distance it was a much more efficient way to keep in regular contact as we worked through the project. So, one key point is keeping that balance of communication. If you can do it in person, that’s fantastic and it’s really good to start, but more important is to have regular communication, and to be flexible in how you maintain communication so that it adapts to what is more appropriate and/or efficient for the users. We were very appreciative of people’s time on this project and particularly for all the clinical input. Surgeons who are working in rural areas—they’re giving their time and their expertise because they love it, because they want to do it. Making sure we can accommodate and adapt to their communication preferences was important, which meant not being too rigid in the ways that one might normally work.
Another thing that I would advise, particularly in health care, is to have a group of people who can provide input. There were a few people who were really strong in this area, and they brought a lot of expertise, but having the opinion of a group really helped to balance the information. For example, there were slight differences in the way people conducted the surgery and they had slightly different opinions on what was needed. There wasn’t a single way of approaching the surgery, so the risk is that if you design to just one person’s specification, it may not be more broadly applicable. It also highlighted areas where you could seek clarification—so and so said this, but you do it a slightly different way: Why is that? Can we understand? It helped to reveal nuances that we might have missed otherwise. That was a really useful lesson for us.
The third aspect is just to understand that the technology is a means to an end. As much as we’re invested in it as engineers, in this context, it’s just one part of a big puzzle. In order to use the technology or for it to be effective, there needs to be people who have expertise in using it, and for training others to use it in the future (Figure 5). In the end, the technology has to fit within a framework, and you have to work with lots of other people to make it successful. You could have the most wonderful piece of equipment, but without the appropriate training or support or infrastructure in place, it could just sit idle.
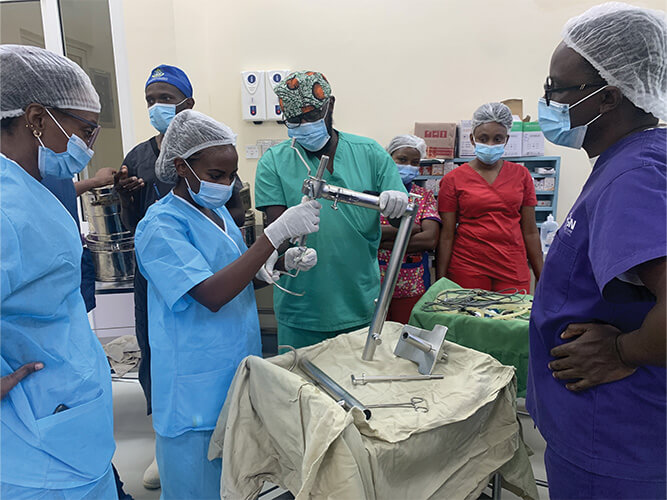
Figure 5. Staff at PECA Hospital (Chogoria, Kenya) assembling the RAIS system. (Photos courtesy of Peter Culmer.)
Development is also part of that big puzzle, where others bring input to the project. When you look to translate a device, you start with ownership spread primarily among the team of engineers and designers and your group of initial stakeholders. When you move to commercialization or others start to use the product, then you have to take a step back. This can be disconcerting at times because you don’t have as much control over it as you did before, but I think you have to let that happen and acknowledge that for the next stage, somebody else has the expertise and the onus to take over.
IEEE Pulse: Any new developments underway for the technology?
Culmer: This article focused on use of the device in India. Since then, it’s gone on to be used in Sub-Saharan Africa. We had identified the potential there because the geography is vast, and the resources are scarce. It’s been really interesting seeing the same device used in these other areas of the world (Figure 6). Also, we recognize that as with any project like this, you receive feedback once it’s implemented. The technology has definitely enabled surgery and that was our primary goal, but now we have a growing list of areas that we could improve, change, and update, which is nice because it shows that people are engaged with it, that they want to help improve the device for the larger community.

Figure 6. Surgical trainers from India and the U.K. visit colleagues at Kabale Hospital (Uganda) to deliver training in gas-less laparoscopic surgery. (Photos courtesy of Peter Culmer.)
One major point is that we worked with our industry partner, XLO (Ortho Life Systems), throughout the development who were fantastic and very supportive. They were really the company that enabled us to translate the tech to get regulatory approval in India and to turn it into something that could be sold and distributed commercially. They have been instrumental in the process of moving this from an academic research study into something that a surgeon can actually buy and receive. Since then, we’ve broadened that relationship. A new company interested in global surgical innovations called Loresa have licensed the technology from the University of Leeds and are working still with XLO to make the device. But the idea is that this new company will supply both the device and training packages, which is a really interesting prospect.
The other area that is particularly important in global health is considering how to make this a sustainable proposition for people to have access to the device long-term. That can be quite challenging because inherently you don’t want to be charging a huge amount of money for things when you’re working in resource-scarce settings, while equally you need somebody to make it and to have a viable commercial setup, so this creates a tension. There is also the need to look at ways where we can deliver training with the device (Figure 7). It’s an interesting approach to join these together as a package and hopefully that model will allow this gasless surgery approach and the use of the device to scale more broadly than we could have achieved separately.

Figure 7. A surgical trainee at Kabale Hospital (Uganda) learning how to assemble and setup the RAIS system. (Photos courtesy of Peter Culmer.)
IEEE Pulse: Can you talk about your next steps?
Culmer: We’re interested now in potential refinements to the system that was developed and reported in the article. In addition, typically you need some other resources to go with that device. For example, in this kind of surgery you need a camera system to look inside the abdominal cavity. These can be expensive to buy, and again, certain localities may not be set up for that kind of surgery. They face either the proposition of purchasing an expensive camera system or trying to find a way to produce a lower cost system. Therefore, in collaboration with other engineering teams (notably the Surgery for All group of Prof. Dankelman at TU Delft—see https://www.tudelft.nl/surgeryforall/ for more information) we’re interested in looking at lower cost imaging devices, but ones that can be used reliably with a good level of quality.
Another thing we’re actively pursuing at the moment is provision of training in low resource settings, working with a great company called Proximie (https://www.proximie.com/). They have a system where you can do tele-mentoring for surgeons, so you can set up an operating theater with a webcam or series of webcams and then have somebody remotely proctor surgeons. Our plan is to explore how to use the system for gasless surgery and help train new generations of surgeons in that area. That’s really exciting because there’s innovation in the technology as well as innovation in the actual training and use of the new systems.
The more you work in the area, you see that there are other challenges, and the goal is to identify which ones are on the critical path—high priority items—and then try to associate funding with those as well, so that you can actually do some work in the area. In some respects, we’re always looking forward to the new engineering challenge or the new technology challenge.

Figure 8. Organizing team of the International Congress on Innovation for Global Surgery (ICIGS 2023), a yearly international conference held by the multidisciplinary Innovations in Global Surgery group (https://surgicalinnovations.org/). (Photos courtesy of Peter Culmer.)
IGS is the global community at the forefront of global surgery solutions (Figure 8), where there is growing enthusiasm across disciplines to develop innovative approaches to improving surgical provision. See https://surgicalinnovations.org/wpp/ for more information.
Acknowledgments
Special thanks to Maulana Azad Medical College (MAMC), India, where we collaborated with surgical colleagues Dr. Anurag Mishra and Dr. Lovenish Bains, who provided their extensive expertise on rural surgery. This was in conjunction with the Association of Rural Surgeons of India and the group of rural surgeons we worked closely with throughout this project (Dr. Biju Islary and Dr. Gordon Rangad to name a few). Also Dr. Jesudian Gnanaraj, a surgeon who is leading the work on gasless surgery in India, for his contribution to development of RAIS and to clinical use and training. Our partners Pd-m International brought their essential medical device design know-how and enthusiasm, while Mr. Sundeep Singh Sawhney and Mr. Tamandeep Kochhar from XLO enabled us to make the leap to a commercial product. Thanks to Medical Aid International (Mr. Tim Beacon) for supporting training and logistics during product development. Thanks to all those in the Global Health Research Group for starting this journey. This work was supported in part by the National Institute for Health Research (NIHR) using U.K. Aid from the U.K. Government to support Global Health Research under Grant 16/137/44 and in part by the U.K. Global Challenges Research Fund (GCRF) allocation to the University of Leeds.
Reference
- M. Marriott Webb et al., “The RAIS device for global surgery: Using a participatory design approach to navigate the translational pathway,” IEEE J Transl. Eng. Health Med., vol. 10, 2022, Art. no. 3700212.
- T. M. Quene et al., “Global surgery is an essential component of global health,” Surgeon, vol. 20, no. 1, pp. 9–15, Feb. 2022, doi: 10.1016/j.surge.2021.10.001.



