Cutting calories, boosting fat-burning with new approaches
More than 30% of the world’s population is overweight or obese. That is double the percentage in 1980, and it is getting worse [1]. That excess weight has been linked to numerous health conditions, notably type 2 (adult-onset) diabetes, the prevalence of which has also nearly doubled since 1980 [2]. Eating less and exercising more is good advice, but it doesn’t work for everyone. Other options such as gastric bypass surgeries and systemic weight-loss drugs are also not suitable for everyone, and can carry risks of their own.
The solution may lie in new technologies to drop pounds by either reducing energy intake or increasing energy expenditure. Three very different approaches are currently under way:
- capsules containing superabsorbent hydrogel made from cellulose and citric acid, which taken before a meal, increase the feeling of fullness and therefore suppress appetite;
- an impermeable, flexible sleeve that lines part of the small intestine, and prevents food from being absorbed along that region of the digestive system;
- a polymer-based nanotherapeutic platform that converts the body’s energy-storing white fat into the energy-burning beige variety, leading to weight loss and improved glucose control.
Premeal capsule
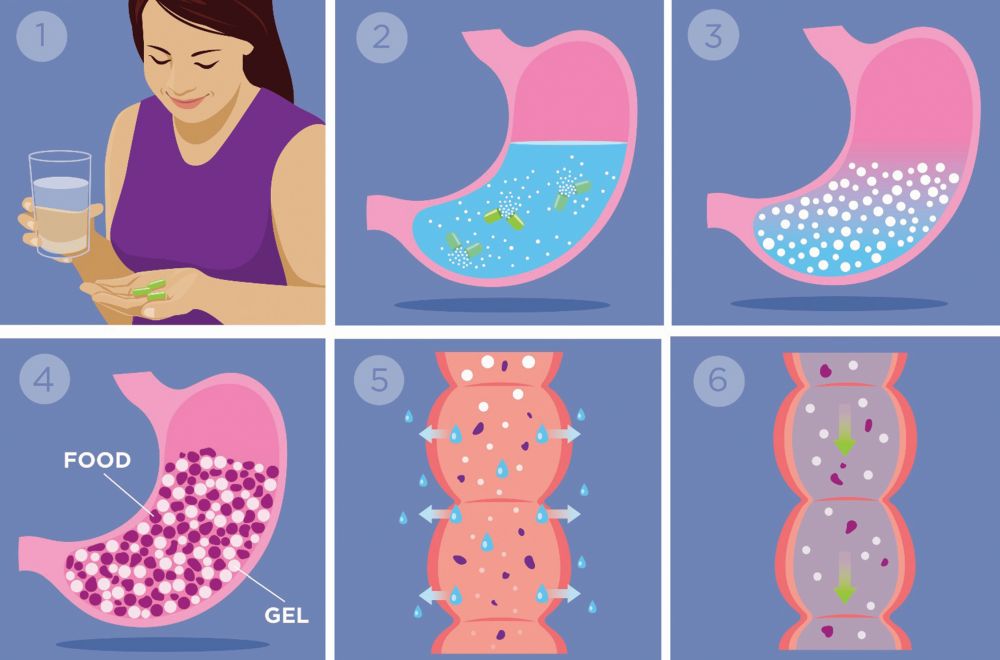
In April 2019, the U.S. Food and Drug Administration (FDA) cleared the capsule, called Plenity (formerly Gelesis100), as a weight-management device for adults who are overweight or have mild to moderate obesity [body mass index (BMI) of 25–40]. The individual takes three capsules with 16 ounces of water at 20 min before a meal, and once in the stomach, the capsules release thousands of hydrogel particles, each about the size of a grain of salt and capable of absorbing 100 times its weight in water or fluid, described Elaine Chiquette, Pharm.D. (Figure 1), chief scientific officer at Gelesis, the Boston, MA, company that is commercializing Plenity.

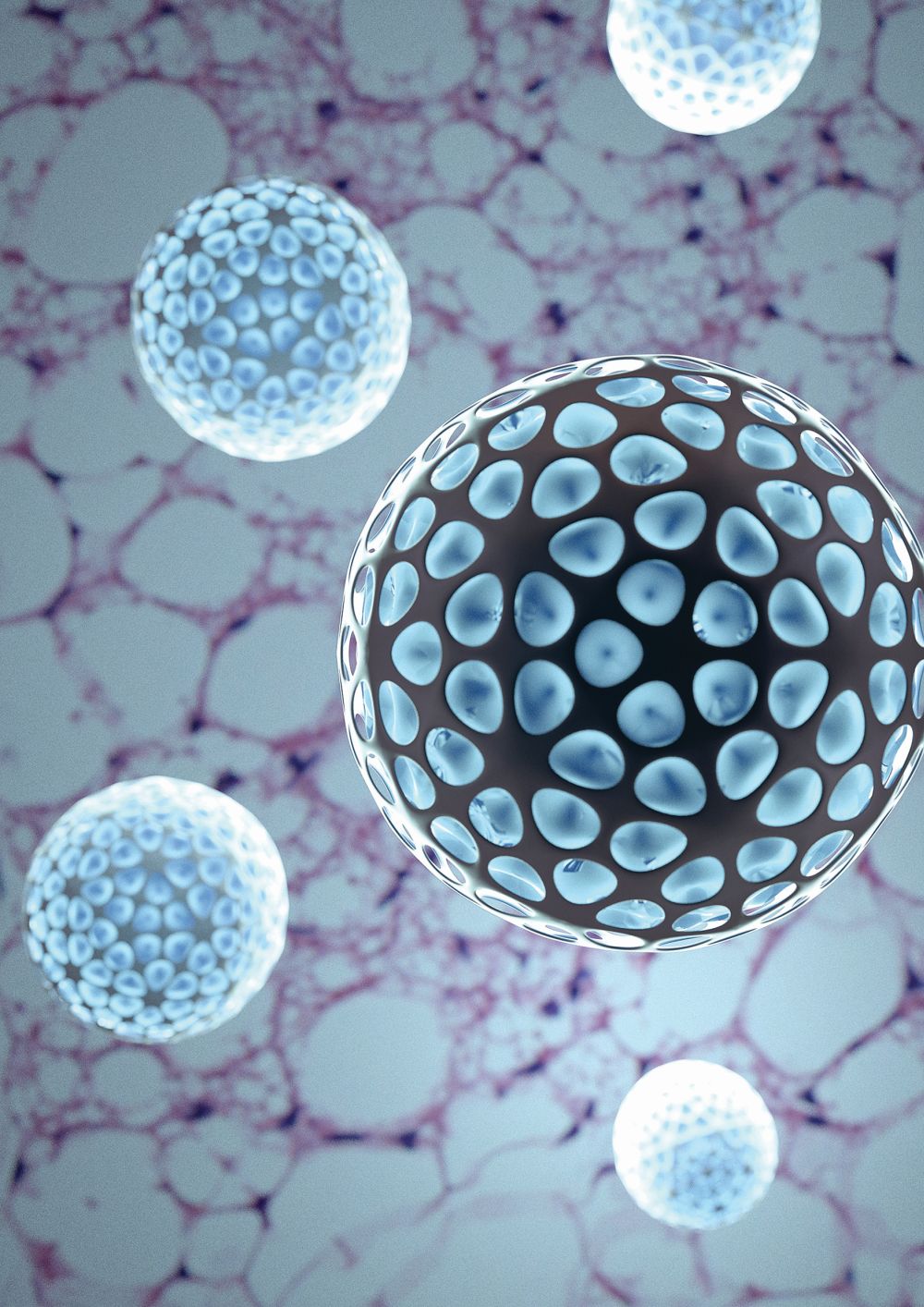
The key to the product is the hydrogel. Originally developed in the lab of materials engineer Alessandro Sannino at the University of Salento in Lecce, Italy, it is composed of cross-linked, naturally derived, food-grade building blocks, namely cellulose and citric acid (Figure 2). As each hydrogel particle becomes fully hydrated, it expands to about the size of a 2-mm bead and maintains that size as it travels from the stomach into the small intestine. “They mix with the meal, so they increase the volume content and the mechanical force that is applied to the gut wall to give the satiety sensation,” she explained. That full feeling helps the patient eat less and lose weight. “This was the first time that someone had actually created a hydrogel that was safe enough to be administered orally but also chronically, so there was a nice fit with a weight-loss indication.”
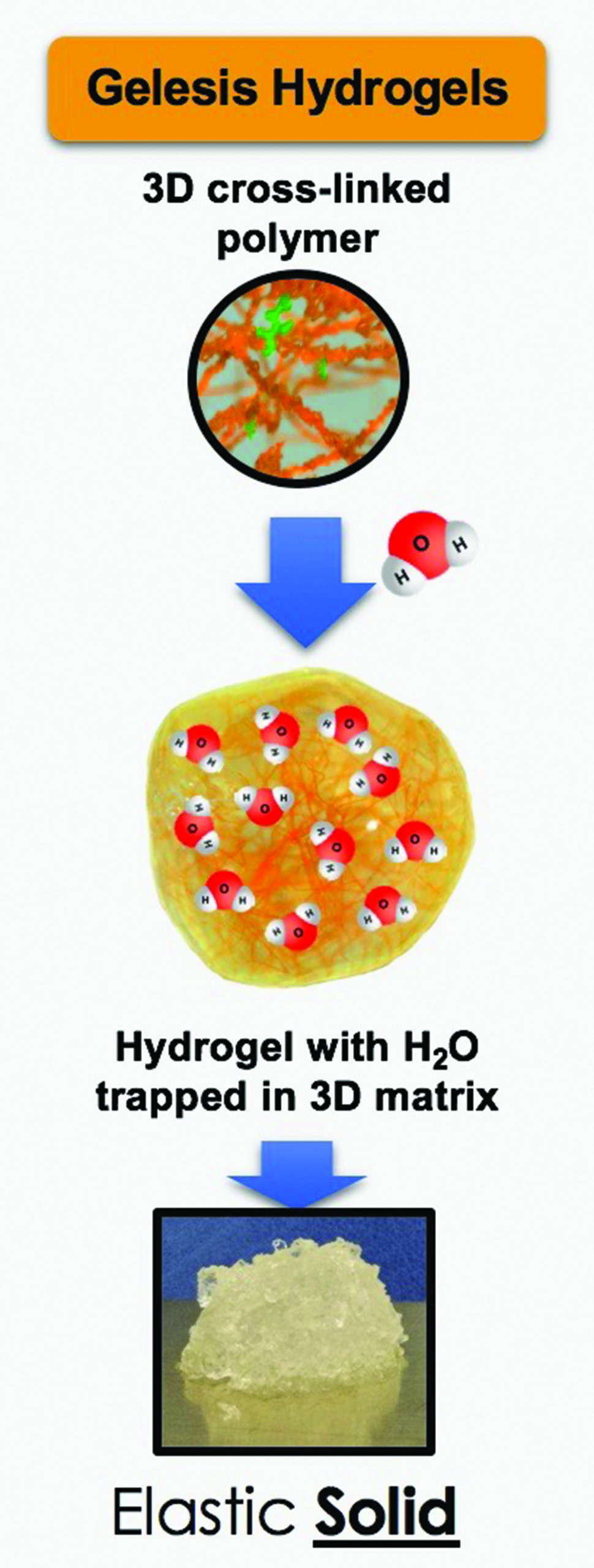
When the hydrogel beads reach the colon, the cross-links are cleaved, the beads open up to release the water, which is absorbed by the body, and the cellulose goes through the bowel movement naturally (Figure 3), Chiquette said. Because the hydrogel particles travel as a 3-D matrix all the way to the colon, she noted, patients not only report eating less at a meal, but also feeling full between meals. At the same time, the beads are negatively charged on the outside so they repel each other. This allows them to easily mix with food rather than forming a mass, “so there is no risk of an obstruction,” Chiquette said.
A pivotal trial [3] of patients taking Plenity at lunch and dinner showed an average of 6.4% weight loss in six months (compared to 2% with a premeal glass of water alone), “and out of the 60% who achieved greater than 5% at six months, they went on to actually lose 22 pounds,” she said.
Gelesis was recently granted the CE mark in Europe, and plans to seek additional approvals in other world markets. Even though the company could have taken an over-the-counter route to commercialize, she hopes the rigorous requirements FDA clearance demands will confer credibility in a market where “there is so much snake oil without much evidence to support efficacy and safety.” A limited, targeted, U.S. launch of Plenity as a prescription product is under way, and once the company scales up its manufacturing capability, it will begin a full U.S. launch in 2021. She added, “We think we can help a lot of people.”
Intestine-lining sleeve
The flexible, fluoropolymer sleeve, developed by GI Dynamics and called EndoBarrier, lines the first 60 cm of the small intestine (Figure 4), preventing food from mixing with digestive fluids and altering hormonal response to food, therefore making the patient feel full longer, reducing caloric intake, and ultimately resulting in weight loss. Used for up to 12 months and then removed, it mimics the effect of the bypass portion of Roux-en-Y gastric bypass surgery, which excises 60 cm of small intestine. EndoBarrier is inserted via a quick, endoscopic procedure, affixed to the wall of the duodenum, and then extended down into the small intestine, according to diabetes clinician Robert Ryder, M.D., of Sandwell and West Birmingham Hospitals NHS Trust in Birmingham, U.K., who has conducted and published numerous studies on the device. “That’s it. It has a little crown anchor at the top. It’s very clever,” he remarked.

Ryder became intrigued by EndoBarrier when he first heard about it at a diabetes meeting in 2011 and saw it as a good option for the many patients who struggle with poor diabetes control and obesity. “Before EndoBarrier came along in my world, the treatment we had for these cases of unresolved diabetes was basically to inject the hormone insulin, which forces the blood-glucose down. If you give a dose and the sugar level isn’t improved enough, the next time you give more, and more, and more,” Ryder described. “The thing about that, however, is that it increases the patient’s weight even further, because insulin works by forcing sugar into the body, which is counter-intuitive, but at that time there was little else we could do.” In addition, the hallmark of type 2 diabetes is resistance to insulin, which means that these individuals tend not to respond to their own insulin “so they often end up requiring gigantic doses of insulin to just scratch the surface,” he said. “That was the mess that we were in.” He also noted that while some medications were used to help with both diabetes control and weight loss, “often they were insufficient.”
EndoBarrier provided an alternative, Ryder asserted. “It didn’t require a knife or a rearrangement of the insides, and it looked like a very simple solution and procedure. It also had the appeal that it wasn’t permanent, so potentially you could bring about an improvement and then put the person back to how they were, hopefully to maintain the improvement.”
Another benefit was its effect on gut-hormone activation, which improves glucose levels. By eliminating digestion along that 60 cm of small intestine, “it basically serves to cause certain increases in the endocrine system that suppress appetite, and other decreases in the endocrine system that stimulate appetite,” Ryder said, noting that while the mechanisms of action are still under study, other changes may also be occurring, such as alterations of the bowel flora or the point at which bile mixes with food.
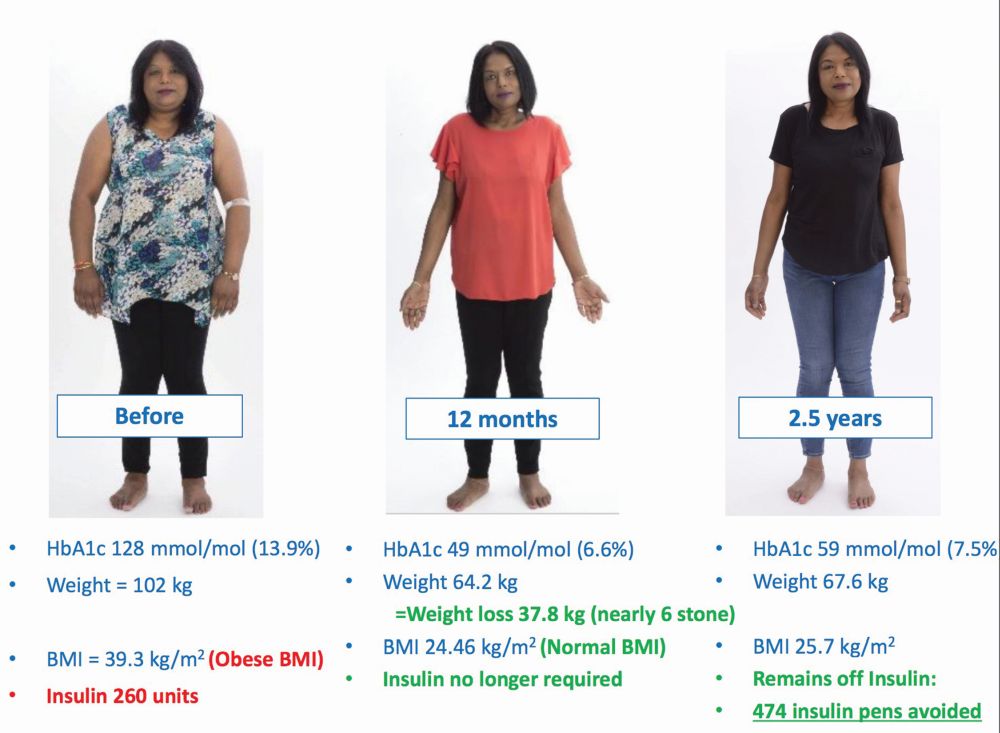
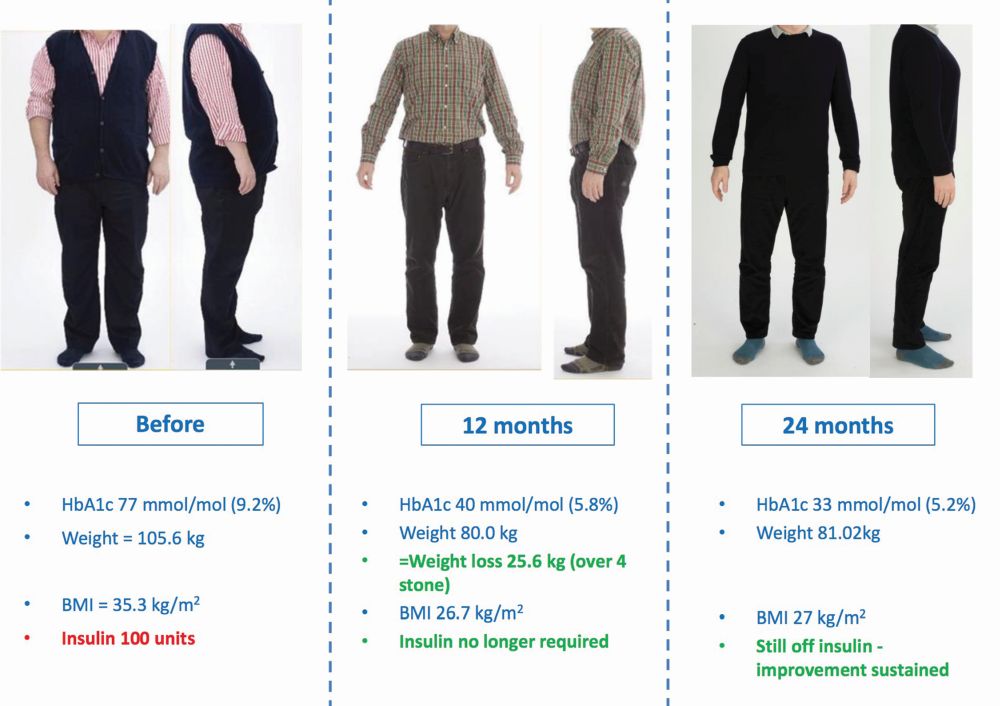
In a 2019 study, Ryder and colleagues followed 62 patients who had EndoBarrier implanted for 12 months between 2014 and 2018. They found that hemoglobin A1C (blood sugar levels) fell by about 30%, weight dropped by approximately 13%, and systolic blood pressure decreased from an average of 138.5 mm Hg to 125.8. Levels of cholesterol and alanine aminotransferase (a marker for nonalcoholic fatty liver disease) also dropped, as did the dose of insulin the patients required, lowering from an average of 100 units to 40 units, with more than 28% of patients able to discontinue insulin altogether [4]. In follow-up work, the researchers also found that 40% of patients managed to maintain the improvements through the year after EndoBarrier was removed; and another 40% remained significantly improved, but not as well as when the EndoBarrier was removed (Figure 5). Approximately 20% “went all the way back to where they started,” Ryder said, noting that many in this group had problems with depression and/or bereavement that likely compromised their success.
In late 2019, GI Dynamics began a randomized and controlled, double-blinded trial of EndoBarrier at five clinical study sites [5], and is now pursuing FDA premarket approval (PMA). It is also seeking to regain the CE mark (received in 2009, but suspended in 2017 due to quality-management-system issues). Ryder is optimistic. “We have lots of data to support EndoBarrier, and I think most diabetes specialists immediately see the appeal of this approach, because there’s nothing else to do now except to increase and increase the insulin, or to go for the full bariatric surgery.”
Fat-cell conversion
Earlier in the pipeline than GI Dynamics’ EndoBarrier or Gelesis’ Plenity, another weight-management/type 2 diabetes tech project is in the works, but to increase energy expenditure rather curb energy intake as a way to reduce weight and manage glucose levels.
The project, based on work done at Purdue University, centers on the two types of fat cells found in adults—white adipocytes that store energy, and the far-fewer-in-number beige adipocytes that burn energy—and developing a way to turn the former into the latter. To accomplish the feat, an interdisciplinary research team has focused on disrupting a particular cell-to-cell communication pathway, called Notch signaling pathway, and by doing so, cause the so-called “browning” of white adipocytes to beige [6]. The team was headed by Shihuan Kuang, Ph.D., professor of animal sciences, and Meng Deng, Ph.D. (Figure 6), assistant professor of biological engineering, biomedical engineering, and materials engineering at Purdue University in West Lafayette, IN. Deng has since founded Adipo Therapeutics also of West Lafayette, IN, to develop, test, and eventually commercialize the technology.

Fundamental to the technology is inhibition of Notch signaling through γ-secretase inhibitors. This has been studied as a systemic treatment for Alzheimer’s disease and cancer, “but by delivering the Notch inhibitors directly to the adipose tissue, there is an opportunity to avoid unpleasant side effects,” said Karen Wurster, chief executive officer of Adipo Therapeutics.
To avoid those side effects, Deng and Kuang developed a bioengineered nanoparticle [7] made of the FDA-approved polymer known as poly(lactide-co-glycolide) or PLGA, which is currently used in sutures and a range of medical devices, and loaded with a γ-secretase inhibitor called dibenzazepine (DBZ) (Figure 7). Their idea is to inject the nanoparticles directly into the white fat, so the polymer carrier can degrade and release DBZ to prompt transformation of browning exactly where it is needed.
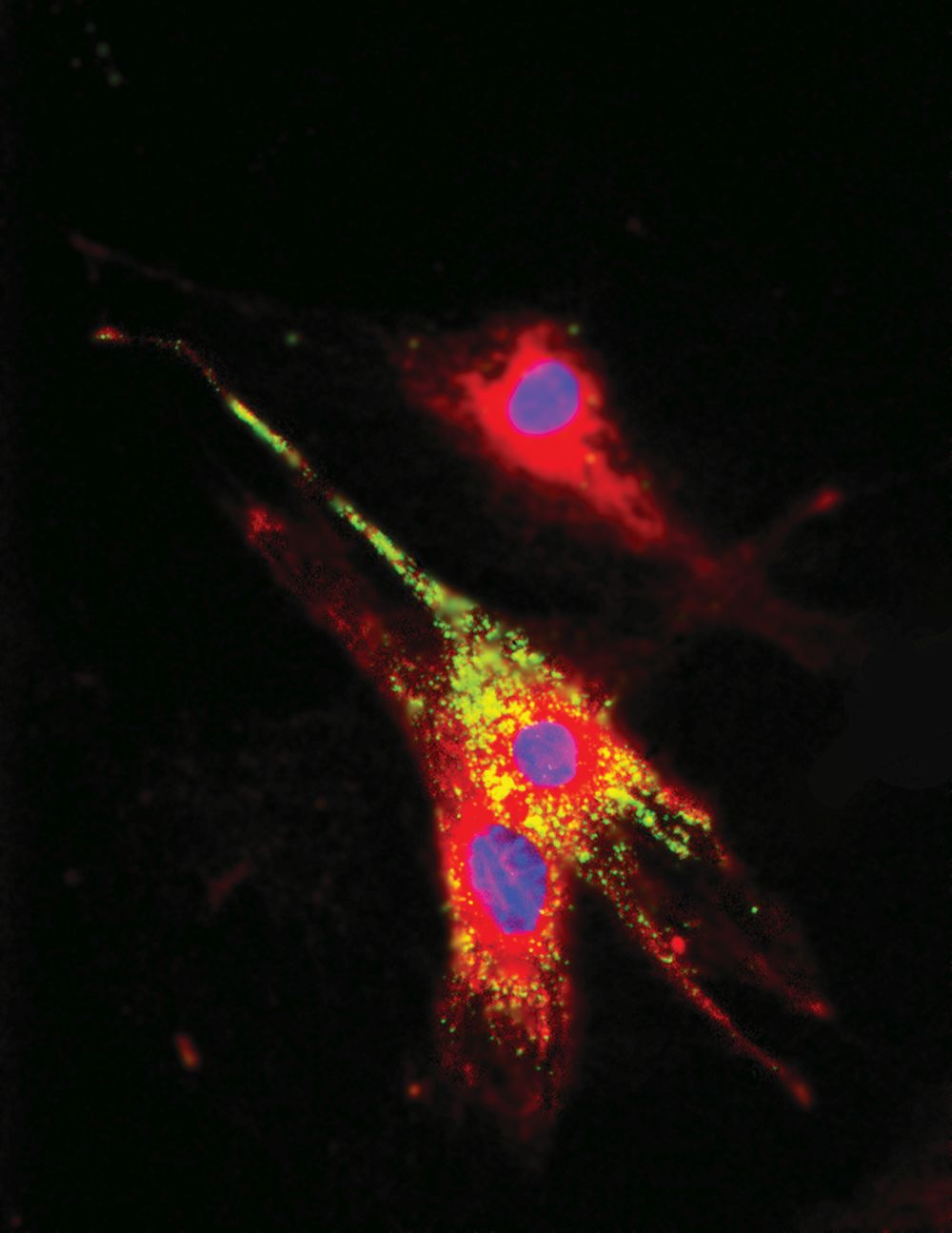
“The proof of concept has already been demonstrated in a number of obese-mice models, including the ability of the nanoparticles to induce the adipocyte browning through the nanoparticle internalization (Figure 8), and also resulting in systemic changes including weight loss as well as improvement of the glucose control,” Deng reported, noting that experiments show diet-induced obese mice experience attenuation of body-weight gain and approximately 54% reduction in glucose level with once-a-week injections of the nanoparticles. More recent large-animal studies in pigs demonstrated similar induction of beige-specific gene expression and reduced subcutaneous adipose tissue expansion [8].
Deng and Wurster hope to soon receive National Institutes of Health Small Business Innovation Research (SBIR) phase II grant funding to support preclinical trials. In humans, the primary target population is type 2 diabetes patients who are overweight. Again, they anticipate using direct-to-fat, localized, nanoparticle injections, Wurster said. “The details will be worked out in development as we analyze through the dose ranging results, with the goal is self-injection, one injection per week.”
Answering the plea
All three of these approaches have the same goal: to improve health for overweight and/or diabetic patients.
“I have been in the field of diabetes forever, and we’ve always had the same problem,” Ryder said. “The standard route of treatment for diabetes is to advise people to have a healthy diet, lose weight, and do more exercise, but people in this situation find it extremely hard to do, and even if they have some success, it is very difficult to maintain it day in and day out all of their lives.”
While some medications developed in the mid-2000s helped lower blood-sugar levels and excess weight, they did not help everyone, Ryder remarked. “We have a worldwide pandemic of type 2 diabetes with the numbers increasing year on year, so even if you manage to have success with large numbers of patients, you are still left with large numbers that don’t have success and (suffer from) complications, such as heart attacks, strokes, damage to the back of the eye and blindness, damage to the kidneys and renal failure, damage to the nerves and blood vessels which can lead to ulceration in the feet and ultimately amputation, so these are all the things that drive us to try and help.”
Deng agreed about the severity of the problem and the need for new solutions. “Obesity represents a significant health care challenge. According to U.S. Centers for Disease Control and Prevention, the prevalence of obesity rose to 42.4% in 2017–2018 [9], and obesity can lead to a number of comorbidities and conditions including diabetes, heart disease, stroke, and cancer, which really represent some of the causes of preventable death. That’s why we’re interested in the area and want to see what we can do.”
References
- GBD 2015 Obesity Collaborators, A. Afshin, et al., “Health effects of overweight and obesity in 195 countries over 25 years,” New England J. Med., vol. 377, no. 1, pp. 13–27, Jul. 6, 2017. Accessed: Jun. 13, 2020, doi: 10.1056/NEJMoa1614362.
- World Health Organization, “Diabetes: Key facts,” Jun. 8, 2020. Accessed: Jun. 13, 2020. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/diabetes
- F. L. Greenway et al., “A randomized, double-blind, placebo-controlled study of Gelesis100: A novel nonsystemic oral hydrogel for weight loss,” Obesity, vol. 27, no. 2, pp. 205–216, Feb. 2019.
- R. E. J. Ryder et al., “The United Kingdom’s first NHS EndoBarrier service for advanced diabesity: 1-year outcomes for all 62 treated patients,” Br. J. Diabetes, vol. 19, no. 2, pp. 110–117, Dec. 2019.
- GI Dynamics, “First patient enrolled in the US STEP-1 clinical trial of EndoBarrier at Michigan medicine,” press release, Jan. 27, 2020. Accessed: Jun. 13, 2020. [Online]. Available: https://gidynamics.com/2020/01/27/first-patient-enrolled-in-the-us-step-1-clinical-trial/
- C. Jiang et al., “Dibenzazepine-loaded nanoparticles induce local browning of white adipose tissue to counteract obesity,” Mol. Therapy, vol. 25, no. 7, pp. 1718–1729, Jul. 5, 2017.
- D. Huang, M. Deng, and S. Kuang, “Polymeric carriers for controlled drug delivery in obesity treatment,” Trends Endocrinol. Metabol., vol. 30, no. 12, pp. 974–989, Dec. 2019.
- D. Huang et al., “Nanoparticle-mediated inhibition of Notch signaling promotes mitochondrial biogenesis and reduces subcutaneous adipose tissue expansion in pigs,” iScience, vol. 23, no. 6, p. 101167, Jun. 26, 2020, Accessed: Jun. 15, 2020, doi: 10.1016/j.isci.2020.101167.
- U.S. Centers for Disease Control and Prevention, “Adult obesity facts,” Feb. 27, 2020. Accessed: Jun. 15, 2020. [Online]. Available: https://www.cdc.gov/obesity/data/adult.html



