When she was 37, Clare developed a tremor down her left side. At 39, she was diagnosed with Parkinson’s disease and put on a series of medications. These helped for a time, but the effect didn’t last. Within a few years, her tremors had grown so severe that Clare was dropping food at her waitressing job. She couldn’t seat guests, and she burned herself when she tried to help out in the kitchen. Increasingly unable to support herself and at a loss for other options, Clare began to look into something called deep brain stimulation (DBS).
DBS is a neurosurgical procedure used for certain refractory diseases where conventional therapeutics aren’t enough. In a typical setup, surgeons thread a pair or more of electrode-tipped probes into precise locations deep inside a patient’s brain where they deliver a steady, unnatural beat of electrical pulses designed to disrupt the pathological signaling of the disease. DBS is relatively well established for certain movement disorders, like Parkinson’s, essential tremor, and dystonia. When it works, it can be astonishingly effective, providing relief in patients for whom literally every other option has failed.
These days, DBS is riding high on a wave of popularity unseen in neurosurgery for over half a century. It’s no longer just for movement disorders but for psychiatry, possibly even general medicine. Start-ups and major device makers have picked up on the potential market, and a new generation of neurodevices awaits just over the horizon. All in all, expectations are very high at present, and that puts DBS in a frankly awkward position.
Clare’s surgery lasted approximately seven hours, followed by a series of three more sessions during which her surgeons adjusted the parameters of the pulses—frequency, amplitude, and timing—to suit her symptoms with a minimum of side effects. It worked. “I’m able to work out front at the restaurant again,” she says in her patient story posted on Medtronic, her device maker’s Web site. “I haven’t felt this good in ten years. People I haven’t seen in two or three years just can’t believe the change.”
Making Headlines
The modern era of DBS got its start at the University Hospital in Grenoble, France, in 1987, when neurosurgeon Alim-Louis Benabid discovered that he could relieve the tremors in his Parkinson’s patients with electrical stimulation to a part of the brain called the thalamus. He wasn’t the first, by any means; neurosurgeons had been quietly using electrical stimulation in the brain for decades, first as a guide for ablation therapies, in which select areas are destroyed, and then as a therapeutic in its own right. But Benabid discovered this fact at a time when Parkinson’s disease had just become quantifiable—animal models of the disease had been created only a few years earlier, and comprehensive new clinical assessment tools for Parkinson’s had just arrived. This made DBS’s effects objectively measurable for the first time, and it was a crucial step needed to move the therapy past regulators. From that point, the practice took off. Its reversibility and adaptability compared to ablation appealed to neurologists, and over the course of the following decades, DBS was approved by regulatory bodies in the United States, Europe, Canada, Australia, and Japan for essential tremor and Parkinson’s. Europe has also granted CE Mark approval for epilepsy and obsessive-compulsive disorder (OCD), and the United States has granted Humanitarian Device Exemptions for DBS in OCD and dystonia. Today, more than 100,000 patients worldwide have been implanted with brain-stimulating devices.
In the past 15 years, DBS’s success in movement disorders has combined with a fundamental shift in thinking across neuroscience that reframes both neurological and psychological diseases in terms not of chemicals, but of disordered neural circuits that span the brain. Find the right hub in the disease circuit, the thinking goes, and you can override the abnormal patterns causing the symptoms. In the last decade, this has opened the potential applications for DBS into everything from depression to Alzheimer’s disease, OCD, addiction, obesity, anorexia, tinnitus, bipolar disorder, Tourette’s syndrome, posttraumatic stress disorder, autism, hypertension, and even urinary function. Nearly 40 different targets in the brain have been tried, and, at the end of 2012, 93 DBS clinical trials of various sizes and phases were underway.
This expansion also makes DBS a powerful tool with which to explore the brain. “Deep brain stimulation offers you a model enabling research on live humans,” explains Marwan Hariz, a neurosurgeon at University College, London. “You can switch it on and off, you can manipulate the stimulation parameters, you can give sham stimulation, you can do many things in a more or less ethical manner to learn more about the brain.” With every surgery, researchers can record signals from neurons deep within the brain, shedding light on how different neural circuits interact and how disease of the mind might manifest in the brain.
And of course, throughout all of this, miracle stories have flourished in the lay press and science literature alike. There’s the transformation of a violently self-destructive autistic boy in Germany into a child able to speak, enjoy car rides, and make eye contact as well as the complete cessation of tics in a Tourette’s patient for whom 42 different medications had failed to make a dent. “Put aside the crossword puzzles and push the ginkgo biloba pills to the back of the shelf,” begins an article in Time, reporting on a seven-person study that found some spatial memory improvement following DBS in epileptics.
Hopes are high, but that’s a problem. Despite the hype and the impressive clinical results, DBS is a nascent field, and very fundamental questions remain unanswered. “We are still struggling after 25 years of deep brain stimulation to know which Parkinson’s patient is suitable or not, how to avoid speech problems, how to avoid balance problems, which kind of dystonia will respond to deep brain stimulation, where is the best target, when to operate,” Hariz says. And that’s in established conditions, like Parkinson’s and dystonia, he explains, where the neurocircuitry is comparatively well mapped. Psychiatric diseases, with their heterogeneity of symptoms and lack of viable animal models, are far less understood.
Outside of movement disorders, much of the data still comes from case studies and small, unblinded clinical trials scattered across that multitude of brain regions and diseases, which has made it difficult to aggregate the data into a cohesive picture. And unlike drug trials, where the mechanism and pharmacokinetics of a therapy are fairly well-established before human trials start, DBS research has been with humans more or less from the start. “We’re acting like it’s phase-three research,” says Joseph Fins, a physician and medical ethicist at the Weill Cornell Medical College, New York. “But in many ways, it’s also like basic science research.”
Dashed Hopes
Late in 2013, news trickled out that one of the largest and most promising phase-three multicenter trials on the use of DBS for treatment-resistant depression had failed a futility analysis. The study had been based on work by Emory neurologist Helen Mayberg, who, in 2005—then at the University of Toronto—published one of the first papers investigating the use of DBS in treatment resistant depression. She’d targeted the white matter—axon tracts—in an area of the brain set deep behind the forehead, called the subgenual cingulate cortex (SCC, also known as Brodmann Area 25). Functional imaging had shown that the SCC tended to be hyperactive in severe depression, and when Mayberg and her Toronto colleagues Andres Lozano and Sidney Kennedy began performing case studies, they found that they could reduce the severity of depression in around half of her patients—people who had failed to respond to everything else.
The BROADEN trial, as it was called (short for BROdmann Area 25 DEep brain Neuromodulation), was initiated by St. Jude Medical, a prominent device maker second to Medtronic in this area. After promising preliminary results, the trial won U.S. Food and Drug Administration (FDA) approval in 2011 to expand to include up to 125 patients in 20 centers. Many believed its foundation on Mayberg’s widely renowned work made it the most viable such trial so far—certainly, the SCC is among the most studied of the DBS candidate sites for depression to date. So when it failed, it hurt.
Disappointing, yes. But maybe not surprising. Last spring, a meta-analysis of the DBS SCC research showed that despite the fact that it is well studied compared to other candidate brain regions, still, only four studies, mostly open-label and with a combined total of 66 patients, had met the authors’ required criteria for inclusion. And while the data did show a real effect of the therapy, 12-month response and remission rates were still only 39.9 and 26.3% (although one study accounted for much of the heterogeneity in remission rates and when those data were dropped, that figure rose to 32.4%). That’s better than other treatments, but it leaves a lot of nonresponders.
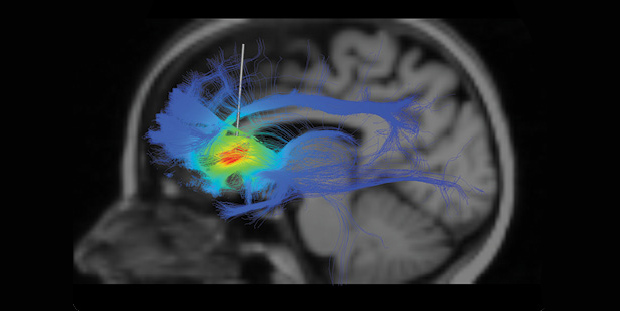
More to the point, however, Mayberg has continued to explore the depression circuitry to investigate not only how DBS helps but why it so often doesn’t. Recently, she has collaborated with Case Western Reserve University bioengineer Cameron McIntyre in a series of fiber-mapping analyses in patients whose depression did or did not respond to DBS (above). The key difference between the groups was not that the probe had been perfectly placed in the SCC—it was whether or not it hit a nexus of three very specific bundles of nerve fibers within the SCC, a nexus that varies from one person to the next and does not have a set of specific coordinates for which a neurosurgeon can simply aim.
This work never informed the St. Jude’s trial of course—it came far too late for that, and Mayberg herself, as a consultant for St. Jude, couldn’t participate in the trial due to conflict of interest anyway. At the moment, it is unclear why the trial failed; the company is working with its physician advisory group to analyze its data now and expects to publicly release the information at a later date. But knowing of Mayberg’s most recent work, it would have been almost more of a surprise had it succeeded. “My own data say close is no cigar,” says Mayberg. “If you only have two out of the three bundles, you will not get that person to the finish line.”
Walk the Line
McIntyre’s Case Western lab specializes in exactly the sort of deep analysis that aims to clarify how to optimize DBS therapy. Using neuroimaging data from DBS patients, he creates individual computational models that combine the placement of a probe with models of the electric field flow and individual neurons. As the number of cases for a given DBS treatment grow, he says, he has been able to begin identifying which stimulated pathways lead to successful treatments and which don’t. This has allowed him to help researchers, like Mayberg, optimize electrode placement. But it also requires that some past surgeries have failed. “It’s the chicken and the egg,” McIntyre says. “You have to have the data to be able to build the models, but if you don’t have the models, it’s hard to optimize the therapy.”
Is it ethical to open someone’s head and put probes in their brain when you know that you don’t know if you’re giving them the best possible treatment? Is it ethical not to, when that patient has a life-destroying condition that nothing else can treat? Risks are rare but can include stroke, infection, speech disturbances, and personality change.
Some studies have investigated whether patients ill enough for the procedure even have the capacity to give consent to something like DBS, particularly given the presence of hype and miracle stories in the media. A 2012 article led by a team at Columbia University, New York, found that severely treatment-resistant depression patients show good performance in measures testing their ability to decide, but some labor under certain therapeutic misconceptions—they underrate the risks to benefits, for instance, and are often under the impression that the point of the procedure is to help the patient, not to explore the efficacy of an experimental procedure.
The danger here is that DBS could move too fast, too far ahead of the data, garner too much hype, too many failures, and crash and burn before it gets anywhere near its clinical and exploratory potential. This has already happened once before: the prefrontal lobotomy of the mid-20th century also enjoyed massive popular support from the media as it swept through psychiatry, despite a shaky scientific foundation. In the end, the procedure’s abuses sparked a public and political backlash that so vilified the practice that it—along with the more cautious neurosurgical practices of the time, like ablation therapy and early forms of DBS—was all but abandoned by the middle of the 1970s.
“We can sometimes ruin surgeries by getting in too quickly,” says Jerrold Vitek, a neurologist at the University of Minnesota. As an example, he refers to a slide he sometimes uses in presentations. On one half, it shows a patient with a frontal lobotomy—too fast?—and on the other half is a patient with Parkinson’s and a child contorted with dystonia—too slow? “The field has been really full of situations where someone does something, it works or not, and if it works, everybody jumped on board and we figured it out later,” he says. “It’s a tough line to walk,” he admits, “but that’s also something that has helped moved the field forward.”
Between a Rock and a Hard Place
There is also a disconnect between the dual realities of DBS’s clinical and financial successes in some parts of medicine, and its immaturity in others. It is simultaneously a therapy done in humans and a tool used for basic science discovery. It has already helped tens of thousands with Parkinson’s, but is clinically unproven for most of its potential applications. There have to be large clinical trials because those are the only kind with enough statistical power to provide clear enough evidence in support of the safety and efficacy of an intervention—but they have to come at the right time.
Weill Cornell’s Joseph Fins has written extensively on such issues. As he sees it, the heart of the problem is the fact that devices that go into a patient’s brain need to be crafted so precisely that only a few industry manufacturers can make them. That pushes alliances between investigators and industry early in the game and lets the companies set the clinical agenda for their probes in contexts that are still only partially understood.
“When you compel people to turn these interventions prematurely into therapies, or you do studies where you don’t collect generalizable data, you’re losing a lot of information,” he explains.
Fins points to the Bayh-Dole Act of 1980, a piece of U.S. legislation that requires intellectual property developed with federal funding to revert back to the institutions that created it. The law was designed to help foster biotech development by giving the investigators and universities the ability to work directly with the needed pharma and device industries to help get ideas to market sooner. In many ways, this has succeeded—look at the booming U.S. biotech industry, for example. But in DBS, where the line between market and discovery is so blurred, Fins believes that the act has led device makers to commodify their investments too soon.
He recommends three key revisions to the law. First, delay the transfer of intellectual property until after efficacy has already been fully established. Second, establish a federal clearinghouse for industry-supplied devices for research so that investigators can access the technology without linking themselves to a given company for preliminary work. This, says Fins, would take the pressure off investigators and free them from early conflicts-of-interest issues that could prevent them from leading a trial that they were best prepared to direct when their expertise was needed most because they’re already connected with the device maker. Industry would still be involved, of course—just a little more distantly, with government entities acting as a buffer. The third revision would tie the clearing house to a formal methods and outcomes registry for every DBS procedure that results, from clinical trial right down to single case study, so that researchers could begin to survey the practice as whole and aggregate results and adverse events.
There are challenges here: A given DBS surgery can cost upward of US$50,000, not counting indefinite follow-ups for the patient to change the pulse generator battery or troubleshoot side effects. Should DBS be funded as medical research? As exploratory science research? It’s in a gray area, and right now, researchers are aided by an inadequate hodgepodge of support from government, nonprofit, and industry—industry dollars are again especially needed for very large trials but can create potential conflicts of interest. But Fins has ideas here too, such as a public–private partnership led by the Foundation for the National Institutes of Health (NIH) that could raise funds from the various potential stakeholders—National Institute of Mental Health institutes, the National Science Foundation, and industry—into a precompetitive consortia of sorts that again helps buffer the scientists from the companies directly.
More daunting perhaps is the fact that changes to this extent require time and collaboration among so many different players, and legislative inertia is no small thing. Still, Fins is optimistic. “I don’t think it needs to be by statutes; I think it can be done in the bureaucracy, through cooperative agreements,” he says. He hopes the recent expansion of interest in DBS might help spur the necessary parties—industry, academia, the FDA, and the NIH—to come together and begin discussing the matter. He points to a series of debates over his ideas that have been waged through the literature over the past few years. “Everybody’s realizing there’s a problem here,” Fins says. “We may not all agree on the solution, but at least we agree there’s a challenge here, and I think that’s a first step.”
Taking Cautious Steps
Of course, there is no real way around the chicken-and-egg problem for patient treatments, except perhaps continuing to accumulate the best information possible. Major initiatives like the European Human Brain Project and the U.S. Brain Research through Advancing Innovative Neurotechnologies may help by spurring new brain mapping tools and insights about the brain.
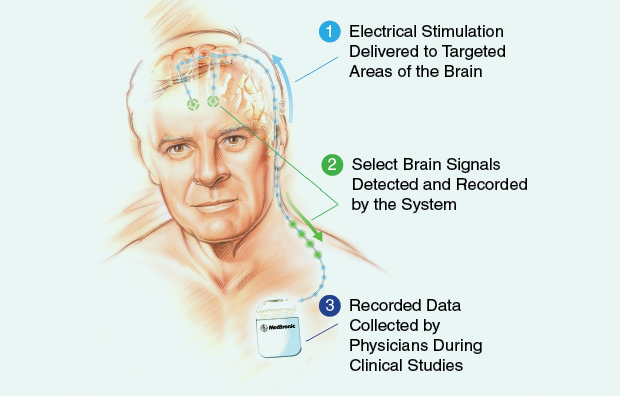
New technologies in DBS may also prove useful, particularly “closed-loop” probes capable of stimulating the brain and recording neural signals while the probe is on or off—keeping the learning window open indefinitely, as it were, or at least until the device runs out of battery power. Medtronic has recently unveiled its closed-loop Activa PC+S system (above) and donated models to a handful of researchers around the world, including Helen Bronte-Stewart, who does Parkinson’s work at Stanford University in California, and Mayberg, who is beginning a small-scale open-label project with the probes to further explore depression. The Mayo Clinic is working on a similar system that will record electrical and chemical information; NeuroPace, a California start-up, has received premarket approval for a probe that monitors the brain for signs of an oncoming epileptic seizure and stimulates preemptively to prevent the attack. If such probes work as planned, they’ll be able to help researchers identify some of the neural signals of a successful treatment and begin to reverse engineer a better understanding of what goes right or wrong in a stimulated brain.
“This technology will have unparalleled possibility to ameliorate the human condition,” Fins says. But to get to that point, DBS—and these major brain initiatives as well—will require a great deal of patience. “I think it’s like cartography and the explorers going across the Atlantic, where every time they hit the western hemisphere, they drew a little bit of coastline, until—as more and more people did this—the map got more involved and eventually the coastlines began to hook up. I think we’re doing much the same thing here, and it’s going to be to this era of discovery what the explorers were in the age of discovery back in the 15th and 16th centuries.”
“If we give it a chance to play out,” he adds.