Over the past two decades, there has been enormous growth in research activity for microwave diagnostic and therapeutic technologies that target the breast. The clinical need for new tools in the breast cancer armamentarium, combined with the promising low-cost, nonionizing nature of microwave technologies, has fueled these investigations. High-fidelity breast phantoms are essential components of computational and experimental test beds for investigating and accurately assessing the performance of new devices, algorithms, and systems related to microwave breast cancer detection and/or treatment.
The ideal breast phantom accurately mimics not only the spatial distribution of the different tissue types in the breast [i.e., skin, adipose tissue (fat), and fibrous/connective/glandular (ductal and lobular) tissues] but also the physical properties of those tissues relevant to microwave interactions with the phantom. Magnetic resonance images (MRIs) of the breast provide detailed information about its anatomical structure, as shown in Figure 1. A large-scale dielectric spectroscopy study of malignant and healthy breast tissue at microwave frequencies reported by the University of Wisconsin, Madison, and the University of Calgary [1], [2] has provided detailed data on the dielectric properties of normal and malignant breast tissue. This structural and dielectric properties information guides the development of both numerical and physical high-fidelity breast phantoms. In this article, we review numerical phantoms for in silico investigations, highlight physical phantoms for experimental studies, and consider the role of phantoms in microwave technologies for breast imaging and sensing as well as breast hypothermia and ablation applications.
Numerical Breast Phantoms
Numerical breast phantoms offer several valuable features that are difficult to achieve with physical phantoms. In particular, they can readily capture the complex heterogeneous interior of the breast (Figure 1) and allow the generation of a large number of test cases spanning the full spectrum of breast volumes, shapes, and fibroglandular density as well as tumor size, morphology, and location.
Phantom Development
Numerical breast phantoms consist of a three-dimensional (3-D) grid of voxels wherein each voxel is assigned relevant physical properties based on the type of tissue it represents. Methods for deriving anatomically realistic numerical breast phantoms (e.g., [3]–[5]) employ the same basic overarching strategy. A spatial map of normal tissue types throughout the breast volume is obtained empirically from breast MRIs of healthy women. MRIs are a preferred source because they provide the tomographic images necessary for creating 3-D models of the breast and also because there is a correlation between the pixel intensity in the MRI and the biological tissue type. Image processing techniques may be applied to the MRI to remove noise and artifacts and to segment the tissue regions. In most cases, interpolation is performed to achieve a small voxel size that is uniform in all three dimensions.
There is greater variation across methods in terms of how dielectric properties are assigned to each voxel in the breast interior. For example, in [3], the voxel intensities were segmented into seven levels. A linear mapping for each level was used to assign dielectric properties to each voxel, with three levels spanning the properties of adipose tissue: one level for transitional tissue (a heterogeneous mix of adipose and fibroglandular tissue) and three levels for fibroglandular tissue. A two-component Gaussian mixture model was used to segment the voxel intensities and generate the linear mappings of each level. In [5], voxels were segmented into three levels, and nonlinear mappings between voxel intensity and dielectric properties were used to assign the properties.
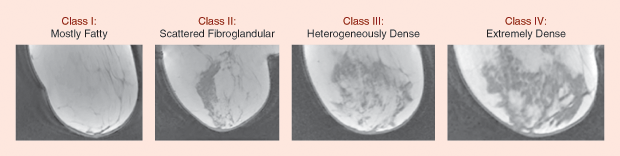
Figure 2 shows representative numerical breast phantoms from the University of Wisconsin’s online breast phantom repository [6]. This repository has nine phantoms categorized according to the American College of Radiology classifications of breast tissue density [7]. All were generated using the procedure outlined in [3] and are presented without tumors.+
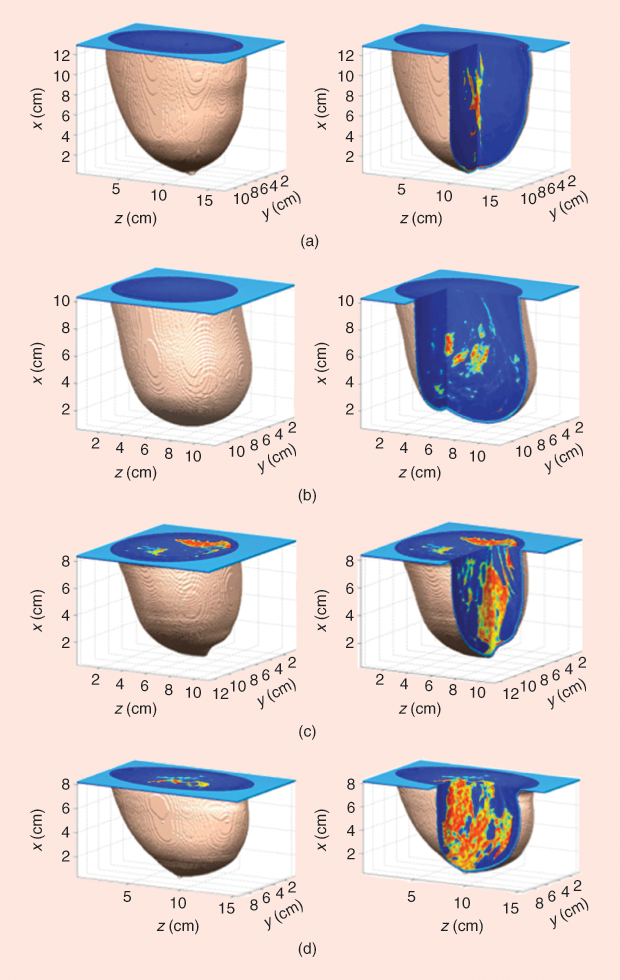
One of two types of tumors may be added to the phantoms. The first type takes the simple form of spherical inclusions. Alternatively, more complex and realistic tumor shapes, such as the smooth, microlobulated, and spiculated tumors presented in [8], may be introduced.
Integration with Commercial Software
Voxel-based numerical phantoms such as those in [6] can be adapted for use in commercial simulation packages. For example, the following procedure is used to import a numerical breast phantom from the University of Wisconsin repository into Computer Simulation Technology (CST) Microwave Studio:
- generate a voxel file labeled with the material numbers for up to 256 different materials
- generate a material file that maps material numbers to material names, nondispersive dielectric properties, and thermal properties
- generate a Visual Basic macro file containing material redefinitions for each of the numbered material types to include frequency-dependent dielectric properties
- import the voxel model into CST Microwave Studio, and then load and run the macro to redefine the various material properties.
The creation of the voxel file, the material definition files, and the macro to apply dispersive properties can be automated with a MATLAB script.
Applications
Realistic 3-D numerical breast phantoms have been widely used to investigate new techniques for microwave imaging and microwave treatments of breast cancer. Examples of microwave imaging algorithms developed and evaluated using simulated array measurements include a variety of inverse scattering techniques for reconstructions of quantitative dielectric properties as well as radar techniques for qualitative reconstructions (e.g., [9]–[13]). Numerical breast phantoms have also been used to investigate microwave-induced thermoacoustic tomography (e.g., [14] and [15]). For these studies, thermal and acoustic properties have been added to the phantoms presented in [3]. Studies of microwave thermal therapies such as hyperthermia treatment of breast cancer (e.g., [16] and [17]) also make use of numerical breast phantoms that include thermal properties. In this latter application realm, numerical phantoms are useful not only for the technology development stage but also for planning patient-specific treatments in clinical settings.
The fact that the dielectric properties of the numerical breast phantom are fully known makes them particularly useful for imaging/ sensing studies, as such ground truth information allows rigorous evaluation of the reconstructed image’s accuracy. Additionally, simulated data acquisition using numerical phantoms is not subject to measurement noise, error, and other nonidealities associated with the complex measurement system, although noise can be artificially introduced into the data. Having full knowledge of the test bed environment is also advantageous in thermal therapy studies, where it is convenient to be able to control the degree of mismatch between the operating environment assumed during the design phase and the environment present in the testing phase.
Physical Breast Phantoms
Clinical studies with human subjects or excised tissues from human subjects provide the most faithful test domain, but performance evaluations are constrained by limited knowledge of the ground truth in these cases. Physical breast phantoms serve as a crucial bridge between studies involving numerical phantoms (virtual patients) and studies involving human subjects.
Physical breast phantoms cannot duplicate the degree of structural realism of MRI-derived numerical phantoms, but they have approximately known dielectric properties that reasonably mimic actual tissue, as well as known structure with moderately complex heterogeneities. Furthermore, experimental data acquisition using physical phantoms introduces the realism of measurement nonidealities.
Development of Phantoms with Dielectric Properties Realism
Tissue-mimicking materials based on custom-made oil/gelatin mixtures (e.g., [18]) exhibit dispersive dielectric properties that can be tuned by adjusting the compositional percentages to cover the full range of tissue types encountered in the breast [2]. Commercially available liquids reported in [19] and [20] have also been shown to offer good dielectric properties realism and improved phantom shelf-life. In general, phantoms constructed from these materials favor dielectric properties realism over anatomical realism.
Physical phantoms with a realistic volumetric shape (e.g., [21]– [23]) may be created by pouring tissue-mimicking materials into breast molds derived from breast MRIs (e.g., [21]) or well-established anthropomorphic molds developed for other breast imaging modalities (e.g., [24]). Breast phantoms with realistic exterior profiles that include a skin layer have been demonstrated in [23] and [25].
However, because the materials in [18]–[20] are prepared in a liquid state and then allowed to cure into a gel, the phantom interiors tend to be fairly homogeneous. Interior heterogeneity can be achieved, but with limited anatomical realism. For example, the heterogeneous environment of the breast interior may be approximated by preparing small chunks of fibroglandular tissue– mimicking gel and then distributing them within a large volume of fatmimicking gel [26], [27]. This achieves heterogeneous distributions, although the specific distribution of the fibroglandular tissue–mimicking material cannot be precisely controlled and does not mimic actual breast anatomy. Interior molds that define a boundary between fat and fibroglandular tissue have been employed to create a more realistic representation of fibroglandular tissue distributions across the four breast density classes [24].
Development of Phantoms with Anatomical Realism
![Figure 3: Representative physical breast phantoms that achieve anatomical realism through 3-D printing of computer models derived from breast MRIs [28]: (a) the side view, showing the exterior shape and (b) the top view, revealing the voids within the plastic volume that are filled with a fibroglandular tissue-mimicking liquid.](https://www.embs.org/wp-content/uploads/2017/07/hagness03-2701489.png)
The advent of 3-D rapid-prototyping printing has allowed for the construction of plastic forms with complex, precisely controlled geometries. This technology has been leveraged to create 3-D printed breast phantoms, such as that shown in Figure 3, with anatomically realistic interior and exterior structure based on breast MRI data [28]. The printed phantom consists of two regions: 1) the plastic structure representing fatty tissue and 2) the voids in the plastic that are filled with a liquid representing fibroglandular tissue. The dielectric properties of the fatty region are dictated by the plastic used in the printer and cannot be tailored. Thus, phantoms of this type sacrifice some dielectric properties realism in exchange for highly realistic spatial distributions of tissue within the breast. Future advances in 3-D printing technology may make it possible to customize the dielectric properties of the printed materials, yielding phantoms that simultaneously offer extremely high levels of dielectric properties realism and anatomical realism.
Development of Phantoms That Mimic Other Properties
Phantoms that sacrifice some amount of dielectric properties realism to achieve other desirable properties have also been proposed. For example, a rubber-based phantom has been developed for investigating microwave imaging systems that utilize compression [29]. The rubber-based material provides enhanced durability and structural flexibility, thereby allowing the resulting phantom to be compressed during data acquisition.
Breast phantoms with realistic thermal properties are desirable for experimental studies of microwave thermal therapies. An overview of possible phantom materials and their thermal properties can be found in [30]. Some of these phantom materials have been engineered to exhibit observable temperature-dependent changes (e.g., color), making it easier to analyze the experimental results. However, it is not straightforward to simultaneously achieve both the desired thermal properties and the desired microwave-frequency dielectric properties. For this reason, ex vivo breast tissue may serve as a preferred test bed over physical breast phantoms in evaluating proposed microwave thermal therapies. A limitation of using either ex vivo tissue or phantoms constructed from static gels as test beds is the lack of blood perfusion—an in vivo effect that plays a role in heat dissipation (e.g., [31]).
References
- M. Lazebnik, L. McCartney, D. Popovic, C. B. Watkins, M. J. Lindstrom, J. Harter, S. Sewall, A. Magliocco, J. H. Booske, M. Okoniewski, and S. C. Hagness, “A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries,” Phys. Med. Biol., vol. 52, no. 10, pp. 2637–2656, May 2007.
- M. Lazebnik, D. Popovic, L. McCartney, C. B. Watkins, M. J. Lindstrom, J. Harter, S. Sewall, T. Ogilvie, A. Magliocco, T. M. Breslin, W. Temple, D. Mew, J. H. Booske, M. Okoniewski, and S. C. Hagness, “A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries,” Phys. Med. Biol., vol. 52, no. 20, pp. 6093–6115, Oct. 2007.
- E. Zastrow, S. K. Davis, M. Lazebnik, F. Kelcz, B. D. Van Veen, and S. C. Hagness, “Development of anatomically realistic nu merical breast phantoms with accurate dielectric properties for modeling microwave interactions with the human breast,” IEEE Trans. Biomed. Eng., vol. 55, no. 12, pp. 2792–2800, Dec. 2008.
- J. M. Sill, T. C. Williams, E. C. Fear, R. Frayne, and M. Okoniewski, “Realistic breast models for second generation tissue sensing adaptive radar system,” presented at the European Conf. Antennas and Propagation (EuCAP), Edinburgh, U.K., 2007.
- A. H. Tuncay and I. Akduman, “Realistic microwave breast models through T1-weighted 3-D MRI data,” IEEE Trans. Biomed. Eng., vol. 62, no. 2, pp. 688–698, Feb. 2015.
- University of Wisconsin Cross-Disciplinary Electromagnetics Laboratory. Numerical breast phantom repository. [Online].
- American College of Radiology, Breast Imaging Reporting and Data System (BI-RADS) Atlas, 4th ed. Reston, VA: Amer. Coll. Radiol., 2003.
- S. K. Davis, B. D. Van Veen, S. C. Hagness, and F. Kelcz, “Breast tumor characterization based on ultrawideband microwave backscatter,” IEEE Trans. Biomed. Eng., vol. 55, no. 1, pp. 237–246, Jan. 2008.
- J. D. Shea, P. Kosmas, S. C. Hagness, and B. D. Van Veen, “Threedimensional microwave imaging of realistic numerical breast phantoms via a multiple-frequency inverse scattering technique,” Med. Phys., vol. 37, no. 8, p. 4210, Aug. 2010.
- T. J. Colgan, S. C. Hagness, and B. D. Van Veen, “A 3-D level set method for microwave breast imaging,” IEEE Trans. Biomed. Eng., vol. 62, no. 10, pp. 2526–2534, Oct. 2015.
- R. Scapaticci, P. Kosmas, and L. Crocco, “Wavelet-based regularization for robust microwave imaging in medical applications,” IEEE Trans. Biomed. Eng., vol. 62, no. 4, pp. 1195–1202, Apr. 2015.
- D. Byrne and I. J. Craddock, “Time-domain wideband adaptive beamforming for radar breast imaging,” IEEE Trans. Antennas Propag., vol. 63, no. 4, pp. 1725–1735, Apr. 2015.
- T. Yin, F. H. Ali, and C. C. Reyes-Aldasoro, “A robust and artifact resistant algorithm of ultrawideband imaging system for breast cancer detection,” IEEE Trans. Biomed. Eng., vol. 62, no. 6, pp. 1514–1525, June 2015.
- X. Zhu, Z. Zhao, J. Wang, G. Chen, and Q. Huo Liu, “Active adjoint modeling method in microwave induced thermoacoustic tomography for breast tumor,” IEEE Trans. Biomed. Eng., vol. 61, no. 7, pp. 1957–1966, July 2014.
- J. Wang, Z. Zhao, J. Song, G. Chen, Z. Nie, and Q.-H. Liu, “ Reducing the effects of acoustic heterogeneity with an iterative reconstruction method from experimental data in microwave induced thermoacoustic tomography,” Med. Phys., vol. 42, no. 5, pp. 2103–2112, May 2015.
- E. Zastrow, S. C. Hagness, and B. D. Van Veen, “3D computational study of non-invasive patient-specific microwave hyperthermia treatment of breast cancer,” Phys. Med. Biol., vol. 55, no. 13, pp. 3611–3629, July 2010.
- P. T. Nguyen, A. Abbosh, and S. Crozier, “Microwave hyperthermia for breast cancer treatment using electromagnetic and thermal focusing tested on realistic breast models and antenna arrays,” IEEE Trans. Antennas Propag., vol. 63, no. 10, pp. 4426– 4434, Oct. 2015.
- M. Lazebnik, E. L. Madsen, G. R. Frank, and S. C. Hagness, “Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications,” Phys. Med. Biol., vol. 50, no. 18, pp. 4245–4258, Sept. 2005.
- S. Romeo, L. Di Donato, O. M. Bucci, I. Catapano, L. Crocco, M. R. Scarfì, and R. Massa, “Dielectric characterization study of liquid-based materials for mimicking breast tissues,” Microw. Opt. Technol. Lett., vol. 53, no. 6, pp. 1276–1280, June 2011.
- N. Joachimowicz, C. Conessa, T. Henriksson, and B. Duchene, “Breast phantoms for microwave imaging,” IEEE Antennas Wireless Propag. Lett., vol. 13, pp. 1333–1336, July 2014.
- A. H. Golnabi, P. M. Meaney, N. R. Epstein, and K. D. Paulsen, “Microwave imaging for breast cancer detection: Advances in three-dimensional image reconstruction,” in Proc. International Conf. of the IEEE Engineering in Medicine and Biology Society, Boston, MA, 2011, pp. 5730–5733.
- D. W. Winters, J. D. Shea, E. L. Madsen, G. R. Frank, B. D. Van Veen, and S. C. Hagness, “Estimating the breast surface using UWB microwave monostatic backscatter measurements,” IEEE Trans. Biomed. Eng., vol. 55, no. 1, pp. 247–256, Jan. 2008.
- A. Mashal, F. Gao, and S. C. Hagness, “Heterogeneous anthropomorphic phantoms with realistic dielectric properties for microwave breast imaging experiments,” Microw. Opt. Technol. Lett., vol. 53, no. 8, pp. 1896–1902, Aug. 2011.
- E. L. Madsen, J. A. Zagzebski, G. R. Frank, J. F. Greenleaf, and P. L. Carson, “Anthropomorphic breast phantoms for assessing ultrasonic imaging system performance and for training ultrasonographers: Part I,” J. Clin. Ultrasound, vol. 10, no. 2, pp. 67–75, Feb. 1982.
- J. Croteau, J. Sill, T. Williams, and E. Fear, “Phantoms for testing radar-based microwave breast imaging,” in Proc. Int. Symp. Antenna Technology and Applied Electromagnetics, Toronto, Ontario, 2009, pp. 1–4.
- M. Klemm, J. A. Leendertz, D. Gibbins, I. J. Craddock, A. Preece, and R. Benjamin, “Microwave radar-based breast cancer detection: imaging in inhomogeneous breast phantoms,” IEEE Antennas Wireless Propag. Lett., vol. 8, pp. 1349–1352, Nov. 2009.
- J. C. Y. Lai, C. B. Soh, E. Gunawan, and K. S. Low, “Homogeneous and heterogeneous breast phantoms for ultra-wideband microwave imaging applications,” Prog. Electromagn. Res., vol. 100, pp. 397–415, 2010.
- M. J. Burfeindt, T. J. Colgan, R. O. Mays, J. D. Shea, N. Behdad, B. D. Van Veen, and S. C. Hagness, “MRI-derived 3-D-printed breast phantom for microwave breast imaging validation,” IEEE Antennas Wireless Propag. Lett., vol. 11, pp. 1610–1613, Dec. 2012.
- J. Garrett and E. Fear, “A new breast phantom with a durable skin layer for microwave breast imaging,” IEEE Trans. Antennas Propag, vol. 63, no. 4, pp. 1693–1700, Apr. 2015.
- A. Dabbagh, B. J. J. Abdullah, C. Ramasindarum, and N. H. Abu Kasim, “Tissue-mimicking gel phantoms for thermal therapy studies,” Ultrasonic Imaging, vol. 36, no. 4, pp. 291–316, Oct. 2014.
- K. I. Ringe, C. Lutat, C. Rieder, A. Schenk, F. Wacker, and H.-J. Raatschen, “Experimental evaluation of the heat sink effect in hepatic microwave ablation,” PLoS One, vol. 10, no. 7, pp. e0134301, July 2015.



