Neurostimulation is an increasingly promising type of intervention for improving and enhancing memory
Across the world, people are living longer. This phenomenon is creating a demographic shift: by 2050, the number of people aged 60 and older is expected to double and the number of people older than 80 is expected to triple [1]. A growing elderly population also means an increase in age-related health concerns. In particular, with age comes normal memory decline as well as an increased risk of Alzheimer’s and other dementias. All this means that now more than ever the world needs interventions to support memory. From developing memory protheses to improving memory consolidation during sleep, neurostimulation is one particularly promising type of intervention for enhancing memory.
Mimicking how memories are made
Robert Hampson, Ph.D., professor of physiology and pharmacology at Wake Forest University School of Medicine, is working on what he calls a “biomimetic model” of using brain stimulation to enhance memory in people who have memory deficits (Figure 1). Hampson and his colleagues are targeting a brain structure called the hippocampus, which plays an important role in encoding new memories. Their goal is to use stimulation as a type of memory prosthesis by mimicking the neural activity that naturally occurs in the hippocampus when a strong memory is formed.

Figure 1. Robert Hampson, Ph.D., professor of physiology and pharmacology at Wake Forest University School of Medicine. (Photo courtesy of Hampson.)
The technique Hampson and his colleagues use is technically a type of deep brain stimulation, which involves delivering electricity directly to the brain through an implanted electrode. However, unlike other forms of deep brain stimulation that typically use high-frequency and fixed-frequency stimulation, their biomimetic stimulation is an asynchronous nonperiodic stimulation.
The team recently tested this form of stimulation in a group of patients with and without a history of head-impact and/or brain injury [2]. These patients already had electrodes implanted in the hippocampus because they were undergoing monitoring for intractable epilepsy. In this study, the researchers tested two different models of neurostimulation: a nonlinear multi-input, multioutput model (MIMO) and a memory decoding model (MDM).
The MIMO model mimics how cells in a specific individual’s hippocampus interact while encoding a memory. Previous studies found that this type of stimulation can restore—and even improve—memory performance [3], [4], [5]. In contrast, the MDM provides stimulation previously shown to help encode certain types of information—in this case, high level categories of images like animal, tool, or plant. In a previous study, this form of stimulation facilitated memory encoding and recall [6]. “The MDM is more specific to the information, and the MIMO is more specific to the normal function of the brain area,” says Hampson.
To test how well the MIMO and MDM stimulation parameters could improve memory in people with brain injury, the researchers used an image recognition task. First, participants were shown an individual image and then asked to identify the image when it was displayed along with six other images—a process designed to help them encode the image. This was repeated for 100–150 trials.
While participants conducted this task, an electrode implanted in their hippocampus recorded signals that could be used to build the MIMO stimulation parameters. The participants later repeated the same task while receiving MIMO or MDM stimulation during two-thirds of the trials. Then, 15–90 minutes after seeing the original images, participants were shown a mix of previously shown images and new images and asked to rate the familiarity of each image.
“Under certain circumstances we could see anywhere from 15% to a 35% improvement in the ability of the person to remember these pictures” when either MIMO or MDM stimulation was applied, says Hampson. He says a limited study found that participants still remembered the images well a day later. Memory improvement was strongest in the patients who needed it the most. “We found that we got a better improvement in performance in the patients who had weaker memory to start with,” says Hampson.
For this task, the MIMO stimulation worked better than the MDM. However, both MIMO and MDM stimulation may end up being useful as memory prosthetics depending on the needs of a particular patient. Hampson says that MIMO stimulation may be useful as a continuous memory aid, like a splint or a crutch. “The MDM model is more, ‘here’s your keys, I’ll hand them to you’,” he says.
The latter may one day allow for customized lifestyle support for a person with memory deficits, for instance from a stroke. It could be used to help people remember specific things, such as whether they’ve taken their medicine or turned off the stove or what they need to get from the grocery store.
Some people, such as those with Alzheimer’s disease, may benefit from the two forms of stimulation being used in concert. “That’s a case where we do think a combination of the MIMO and the MDM would be very effective,” says Hampson.
Hampson is also testing the use of constant, very low frequency (4–7 Hz) deep brain stimulation to see whether it may be effective in improving memory. He says he’s witnessed growing interest in deep brain stimulation from companies, including for the more complicated pattern stimulation his group has been pioneering. “I think we have a number of possibilities here for bringing hope to people who have memory impairments from multiple diseases and disorders,” he says.
Emerging “electroceuticals”
Robert Reinhart, Ph.D., associate professor of psychological and brain sciences at Boston University, and his lab are using a different form of stimulation called transcranial alternating current stimulation (tACS) to try to improve memory (Figure 2). This technique involves applying an alternating electric field to the surface of the scalp via electrodes.

Figure 2. Robert Reinhart, Ph.D., associate professor of psychological and brain sciences at Boston University. (Photo courtesy of Reinhart.)
“It’s a way to safely and noninvasively modulate large-scale brain network dynamics,” says Reinhart. This stimulation can also be applied at specific frequencies at different parts of the brain, which makes it a powerful tool for cognitive neuroscientists like Reinhart. Reinhart is using a high-definition tACS approach with smaller, more numerous electrodes that provide better spatial resolution (Figure 3). “We’re trying to do what we call synchrony modulation, trying to modulate streams of brain connectivity,” he says. “Targeting two or more regions simultaneously to isolate and activate numerous nodes of large-scale brain networks.”
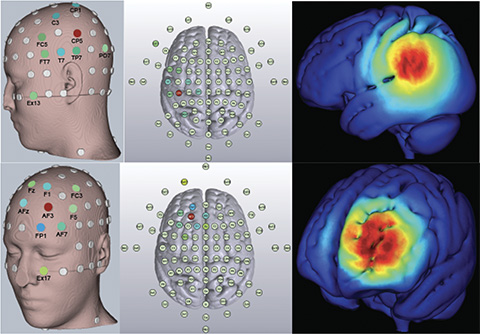
Figure 3. Locations of electrodes and electric field intensity for parietal lobe (top) and prefrontal cortex (bottom) stimulation. (Image courtesy of Shrey Grover.)
Reinhart’s group recently used tACS to enhance working memory and long-term memory in a randomized, double-blind study of older adults [7]. In this study, participants were asked to memorize a list of 20 words while they received 20 minutes of stimulation to either their prefrontal cortex, their parietal lobe, or a sham stimulation for four consecutive days. They were asked to recall the words after hearing each list and also one month later.
Reinhart’s group found that high frequency stimulation of the prefrontal cortex could modulate long-term memory, whereas low frequency stimulation of the parietal cortex preferentially improved short-term memory. The average probability of correctly recalling words rose by about 30% from the first day to the fourth day of stimulation, although some participants saw greater benefits than others. “The older people who had greater cognitive impairment at baseline showed the largest stimulation-induced memory benefits,” says Reinhart.
Reinhart recently received funding for the National Institute on Aging to test the technique with people who have mild cognitive impairment or Alzheimer’s disease. The lab is also working to see how the results of the recent study generalize to other types of memory tasks as well as other cognitive tasks that engage the prefrontal cortex.
Reinhart says that tACS has an attractive safety profile because the stimulation is so low intensity—less than one volt per meter. The device that applies the stimulation is also small, relatively inexpensive, and easy to use. These attributes mean that it could be a future drug-free therapeutic that patients could use at home.
Reinhart says the pharmaceutical industry has taken notice of the potential of tACS as an “electroceutical.” He adds, “But it’s different from drugs because it’s not acting on neurochemistry, which could be brain-wide potentially, but [instead] acts on particular electrical neurocircuits, which could be distributed spatially or confided spatially, depending on what you’re targeting.”
One way that tACS is like a drug is that the “dose” of the stimulation can be adjusted. The dose of the stimulation depends on the specific protocol that is used, including parameters such as electrode number, size, location, stimulation, waveform phase, frequency, intensity, and duration.
Currently, multiple groups are testing out the technique to fine tune the parameters needed to optimally modulate different mental functions. However, some of these researchers have been publishing less rigorous work, such as studies without adequate controls, as Reinhart discovered when conducting a recent meta-analysis on the technique [8]. For Reinhart, it is also important that tACS studies be grounded in what is already known—or theorized—in computational neuroscience. “We’re trying to move away from the ‘poke and hope’ approach,” he says.
Exploring the sleep-memory connection
Maya Geva-Sagiv, Ph.D., project scientist at the University of California, Davis, CA, USA, is using brain stimulation to probe the connection between sleep and memory (Figure 4). In particular, Geva-Sagiv and her collaborators have been exploring how information is transferred from the hippocampus to the cortex during sleep (Figure 5). She says that one can think of the hippocampus as a USB drive that collects experiences throughout the day. “During sleep sessions, that’s your opportunity to download these experiences and pass them to cortical areas, which hold on to the long-term memories and integrate this new information into the memories,” she says.

Figure 4. Maya Geva-Sagiv, Ph.D., project scientist at the University of California, Davis. (Photo courtesy of Geva-Sagiv.)
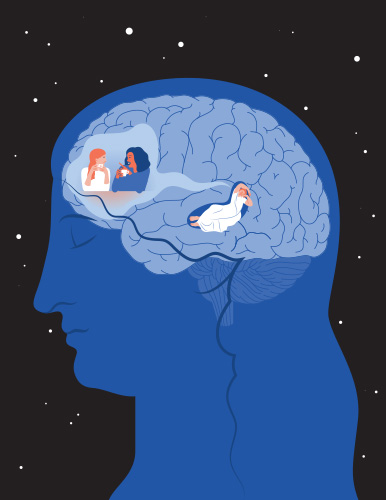
Figure 5. Stylized schematic showing connection between hippocampus and prefrontal cortex in consolidating memory during sleep. (Image courtesy of Maya Geva-Sagiv, by Osnat Feitelson.)
Geva-Sagiv and her colleagues hypothesize that this transfer of information occurs thanks to an intricate dance of coordinated activity in the brain. “The model defines very specific temporal windows where high frequency oscillations called ripples happen in the hippocampus at the exact same time when sleep spindles and slow waves happen in the cortex,” she says. “That opens a window of opportunity to modify information in the cortex based on newly acquired information in the hippocampus. It’s a very special window of opportunity when we have those faraway brain structures synchronized and able to pass information.”
Testing this model in humans has been difficult because the hippocampus is so deep in the brain. A recent study using deep brain stimulation in patients with medication-resistant epilepsy allowed Geva-Sagiv and her colleagues the opportunity to see whether using stimulation to improve synchronization between the hippocampus and the cortex during sleep could improve memory [9].
In this study, participants completed a “celebrity pet” task in the evening where they were asked to try to memorize photos of people’s faces (a recognition task) and associate these people with their pets (an association task). The faces were all celebrities, or doctors and nurses known to the participants, whereas the pets were unknown.
After a brief break, participants were tested on their ability to recognize the previously presented faces—and not falsely recognize newly presented faces—as well as their ability to remember the associated pets. They then went to sleep. Each patient completed two nights of the experiment—one with stimulation and one without.
The next morning, the patients performed the same memory tasks as they had the previous day. While there was only a slight improvement in how well participants remembered the people-pet parings, patients performed significantly better at correctly recognizing previously displayed people—and especially at not falsely remembering new faces—on mornings following stimulation than on mornings following undisturbed sleep. A second group of patients who received stimulation without the precise time-locking did not show improvements in recognition.
Recordings of brain activity during sleep found that the stimulation was effective at increasing the coupling of sleep activity signatures—ripples, slow waves, and spindles—believed to be important in memory consolidation and that this increased synchrony strongly correlated with memory enhancement. “It’s a demonstration that with a very low-amplitude localized intervention, you can have really large effects when it’s done correctly during sleep,” says Geva-Sagiv.
She says the effect was stronger than she anticipated and helps support the idea that cross-brain communication during sleep is important for memory consolidation (Figure 6). This, in turn, may one day help pave the way for a new mode of memory enhancement.

Figure 6. Recent investigations into the sleep-memory connection support the idea that cross-brain communication during sleep is important for memory consolidation. (Image courtesy of Maya Geva-Sagiv, by Osnat Feitelson.)
“I’m very hopeful. It lays the ground for several lines of research that could prove to be useful,” Geva-Sagiv says. “Hopefully, we can find a way to do this based on noninvasive measures—that is the dream. But first, I think establishing what we understand based on the current study is important. It will take a lot more research.”
References
- World Health Organization. Ageing and Health. Accessed: Oct. 13, 2023. [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health
- B. M. Roeder et al., “Patterned hippocampal stimulation facilitates memory in patients with a history of head impact and/or brain injury,” Frontiers Human Neurosci., vol. 16, Jul. 2022, Art. no. 933401, doi: 10.3389/fnhum.2022.933401.
- T. W. Berger et al., “A cortical neural prosthesis for restoring and enhancing memory,” J. Neural Eng., vol. 8, no. 4, Aug. 2011, Art. no. 046017, doi: 10.1088/1741-2560/8/4/046017.
- R. E. Hampson et al., “Facilitation of memory encoding in primate hippocampus by a neuroprosthesis that promotes task-specific neural firing,” J. Neural Eng., vol. 10, no. 6, Dec. 2013, Art. no. 066013, doi: 10.1088/1741-2560/10/6/066013.
- R. E. Hampson et al., “Developing a hippocampal neural prosthetic to facilitate human memory encoding and recall,” J. Neural Eng., vol. 15, no. 3, Jun. 2018, Art. no. 036014, doi: 10.1088/1741-2552/aaaed7.
- X. She, T. W. Berger, and D. Song, “A double-layer multi-resolution classification model for decoding spatiotemporal patterns of spikes with small sample size,” Neural Comput., vol. 34, no. 1, pp. 219–254, Dec. 2022, doi: 10.1162/neco_a_01459.
- S. Grover et al., “Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation,” Nature Neurosci., vol. 25, no. 9, pp. 1237–1246, Aug. 2022, doi: 10.1038/s41593-022-01132-3.
- S. Grover et al., “A meta-analysis suggests that tACS improves cognition in healthy, aging, and psychiatric populations,” Sci. Transl. Med., vol. 15, no. 697, May 2023, Art. no. eabo2044, doi: 10.1126/scitranslmed.abo2044.
- M. Geva-Sagiv et al., “Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans,” Nature Neurosci., vol. 26, no. 6, pp. 1100–1110, Jun. 2023, doi: 10.1038/s41593-023-01324-5.



