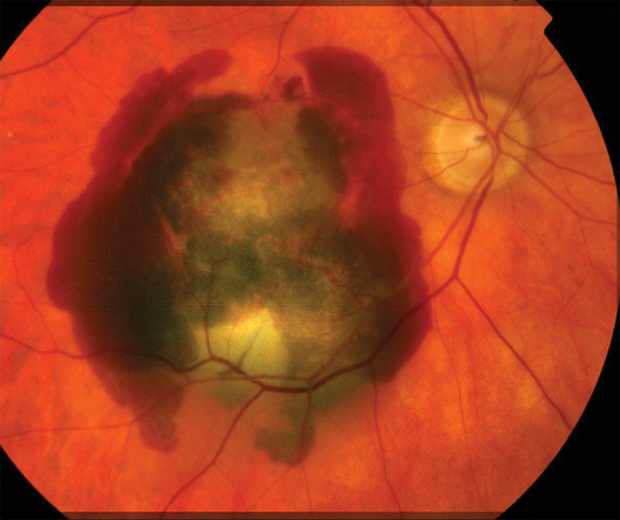
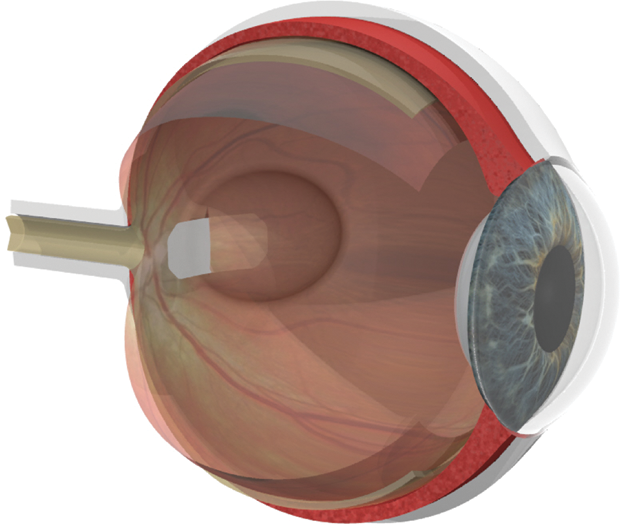
For people who have relied on good eyesight for the first six or seven decades of their lives, the diagnosis of age-related macular degeneration (AMD) is devastating. This disease, which currently has no cure, can severely limit central vision and, in many cases, may culminate in legal blindness (unable to see the Big E on the eye chart; vision less than 20/200). Early results from two new stem-cell trials, however, suggest that this type of treatment may be able to restore sight in people with both the dry and wet forms of AMD. AMD affects more than 170 million people worldwide— more than 11 million in the United States alone—and it is the leading cause of vision loss in the industrialized world [1]. Caused by the deterioration of the macula (the central portion of the retina), it is divided into two basic types: wet AMD, in which abnormal blood vessels develop beneath the retina and macula, and leak blood and serum that raise and compromise the macula (Figure 1); and the much more common dry AMD, in which deposits called drusen may form, and be linked to loss of vision-associated nerves within the macula, a process called atrophy.
AMD affects more than 170 million people worldwide— more than 11 million in the United States alone—and it is the leading cause of vision loss in the industrialized world [1]. Caused by the deterioration of the macula (the central portion of the retina), it is divided into two basic types: wet AMD, in which abnormal blood vessels develop beneath the retina and macula, and leak blood and serum that raise and compromise the macula (Figure 1); and the much more common dry AMD, in which deposits called drusen may form, and be linked to loss of vision-associated nerves within the macula, a process called atrophy.
In dry and wet AMD, the retinal pigment epithelium (RPE) also atrophies. This layer of cells supports the retina’s light-sensitive photoreceptors, and when the RPE layer deteriorates, it damages the photoreceptors, which brings about vision loss. An understanding of this cascade effect led two research groups to focus on replacing the RPE layer, and each felt employing stems cells might be the best way to do it.
New approach for dry AMD

“For the dry form of AMD, there’s some vitamin therapy that is used to slow the progression of the disease, but once people have reached the end stage of the dry form, which is called geographic atrophy, they lose RPE cells, and that causes them to have irreversible vision loss,” explains researcher, physician, and surgeon Amir H. Kashani, MD, PhD, assistant professor of clinical ophthalmology at the University of Southern California (USC) Roski Eye Institute (Figure 2, right). “Our thinking was that by replacing those supporting RPE cells before the overlying photoreceptors are damaged permanently, we might be able to provide the necessary environment for the photoreceptors to become active again and preserve visual function.”
The California Project to Cure Blindness (CPCB) research group, of which Kashani is a member, wasn’t the first to think about using stem cells to reintroduce RPE cells. Others have tried to inject stem-cell suspensions underneath the retina or into the adjacent vitreous cavity of the eye in the hopes that the stem cells would become RPE or at least help the surrounding retina. While those efforts have had limited success in improving vision, Kashani says, “There’s no real long-lasting effect, and they don’t seem to recreate the normal anatomy of the retina. In other words, the cells don’t seem to live and do what they should.”
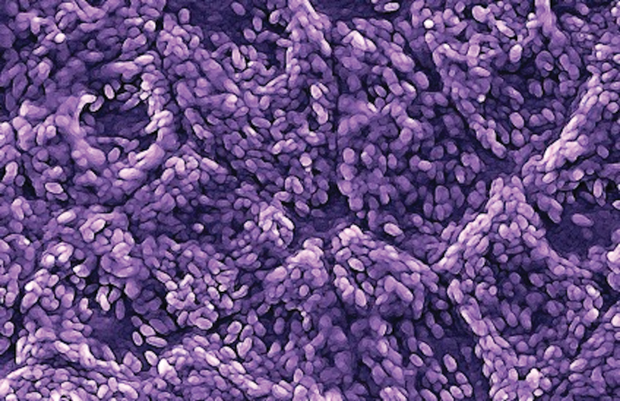
The CPCB group took a different approach. Rather than inject the RPE cells as a suspension, they decided to generate a cell patch that would have the exact anatomical configuration that occurs in a normal retina (Figure 3). The CPCB group—USC, University of California at Santa Barbara, California Institute of Technology, and the Menlo, California, company Regenerative Patch Technologies—brought together a large group of scientists, engineers and clinicians, and came up with a way of turning human embryonic stem cells into RPE, then growing those as a monolayer on a plastic scaffold, and implanting it, he says. The scaffold was critical. “Our studies have shown that by having the cells actually adherent to some kind of substrate, they survive longer, they behave more like native RPE, and they can actually go into the areas where the RPE is missing, because we implant them there directly.”

Development of the substrate was pioneered by CPCB group member Mark S. Humayun, MD, PhD, a biomedical engineer and retinal surgeon, who is the director of the USC Ginsburg Institute for Biomedical Therapeutics and a co-director of the USC Roski Eye Institute (Figure 4, right). Humayun already had another major vision development under his belt: the Argus II Retinal Prosthesis System, a bioelectronic device designed to stimulate the remaining nerve cells in patients with certain types of hereditary vision loss. The Argus II received FDA approval in 2013 [2], and is now available through Second Sight Medical Products, Inc., Sylmar, California.
“That Argus II project,” Humayun comments, “gave us a lot of insight into the surgical procedures and the types of materials that can be tolerated over long periods with the retina, and also into microfabrication.” For the AMD project, in fact, he and his group ended up using a biocompatible material they had employed to microfabricate retinal electrodes.
Called parylene, the material is a monomer that is also used to protectively envelop the wires for some pacemakers and other implanted medical devices (Figure 5). Although parylene is usually grown as a flat coating via chemical vapor deposition, it can also be built into a 3-D structure using photolithography, Humayun says. The 3-D aspect was important for the macular-degeneration project, because they wanted to microfabricate the scaffold to mimic the normal basement membrane, called the Bruch’s membrane, which lies beneath the RPE cells and allows the diffusion of nutrients and other molecules critical to cell survival.
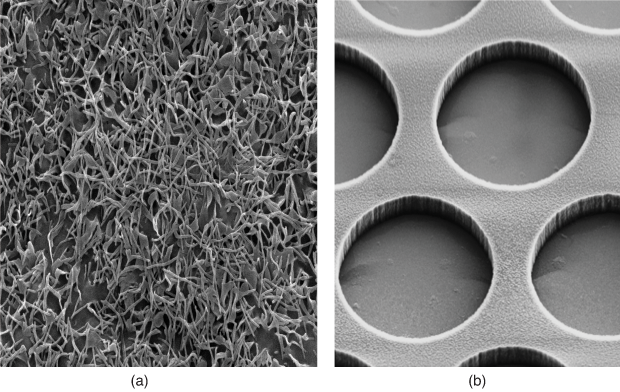
To make a Bruch’s-like scaffold, the researchers tried a perforated design to allow diffusion, but the RPE cells passed right through the holes. “Even if we had just 1-micron holes, these 10-micron or bigger RPE cells would squeeze their way to the other side. I couldn’t believe that was happening, but it showed that we couldn’t have any holes at all,” he explains. In answer, they developed a nonporous scaffold with thick and thin regions to allow diffusion. “It took us 5 or 6 years of work, but lo and behold, we finally did it,” he describes. When stem cells were added and allowed to incubate for about 30 days, they formed a fully mature RPE-cell layer on the membrane that was ready for implantation.
Humayun also designed a surgical tool that could fold the 3.5 × 6 mm membrane patch without tearing, crimping, or creasing it; slide it through an implantation shaft that was about the diameter of a human hair; and then unfurl it once it was in place within the eye (Figure 6).
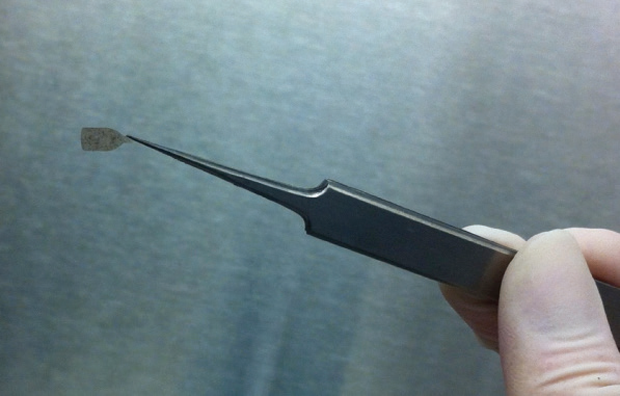
Following successful animal experiments showing that the surgical tool worked and the RPE membrane cells survived for more than a year, the CPCB team (with Kashani as clinical PI) started a pilot trial of the system in 2017. The trial began with five patients who had dry AMD and then added 11 more. The patients underwent a relatively short outpatient procedure to implant the cells and were sent home the same day. The researchers were so excited by the progress made in the initial five patients that they decided to publish those findings in 2018 [3] while continuing to gather data on the remaining 11 in 2019. “In those initial five, one of the patients improved reading on a standard eye chart by about 16–17 letters. And two others actually had positive changes in their retinal function, which we measure by using fixation, or asking a patient to look at a target and measuring how well they lock onto that target. Both were able to do better after the surgery than before,” Kashani says. “Those results were very encouraging, because these were end-stage patients who had geographic atrophy for a decade or more.”
As the pilot-trial data collection wraps up in 2019, a phase II trial of the AMD implant will also begin. Humayun remarks, “We hope that our results will continue to show improvement in visual function in these patients who otherwise are rendered legally blind or soon will become legally blind, meaning they cannot read or write or recognize faces because of dry macular degeneration.”
Stem cells for wet AMD too
For a research group at Moorfields Eye Hospital NHS Foundation Trust and University College London in the UK, the concept for its stem-cell approach grew out of a previously developed treatment for patients who had lost RPE cells to AMD, but still had some of the cells remaining. In this treatment, the macula was lifted, rotated on the optic nerve, and set back down on a bed of healthy cells. It worked, but it was a long and complex operation with a follow-up operation two months later, and given the large number of patients with AMD and their average age, the procedure was simply impractical, says Prof. Lyndon da Cruz, MD, PhD, a retinal surgeon at Moorfields. “What this did show us, however, was that if you put healthy cells under the macula after the vision had gone, you could get the vision back.”
That led them to try something different: Instead of moving the macula to the healthy cells, they decided to bring healthy cells to the macula. “We wanted to develop a cell patch that had the healthy cells we needed; load it into an injector system, which is in fact what happens to the lens in cataract surgery; and develop a very simple operation modeled after cataract surgery and that most or all retinal surgeons could do in under an hour under local anesthetic, which would make it practical for the numbers,” da Cruz says. Called the London Project to Cure Blindness, it kicked off more than a decade ago as a partnership between the research groups of da Cruz and Prof. Pete Coffey, PhD, of the University College London’s Institute of Ophthalmology.
“As biologists, scientists, and surgeons, we were entering a new field because we had to develop an injection system, which was an engineering challenge. We also had to work with new materials to make this membrane on which to grow the cells, so it had to be biocompatible, stable in an engineering sense, and flexible so we could load it into the injector,” da Cruz describes. They designed a prototype sufficient for initial safety trials in animals, and have since brought in engineers to fine-tune it for use in humans.
When all was said and done, the multidisciplinary group had succeeded in converting embryonic stem cells into RPE cells, growing them on an artificial basement membrane, and forming a patch made of the membrane and approximately 100,000 RPE cells (Figure 7). They also perfected an injection system that could take the rolled-up patch and deliver it into the eye through small incisions. Each component of the project had its separate challenges, but da Cruz says it all came together well because they were able to tackle the components in parallel, rather than one at a time.
“Ordinarily, these projects would be running serially, so first you’d get some cells, then you’d grow them on a membrane, then you’d make an injection system, etc., and this of course strings the time frame out to inconceivably long periods,” he comments. By working in parallel, however, “we were developing the tool while we were choosing the membrane and while we were developing the cells and while we were coordinating the operation, so we could not only compress the time frame, but it also allowed us to know what limitations or boundaries we had.” For example, he says, they knew the constraints and capabilities of the injector system and the requirements of the membrane, so they could modify one or the other, or both, “so that everything came together in a practical way at the same time.”

In 2017, the research group conducted an initial trial of the system on two patients who had wet AMD and had lost the ability to read, even with eyeglasses (Figure 8). In the results published earlier in 2018, the group reported that both patients had regained vision sufficiently well that they could read again [4]. “We have two with good outcomes and good safety profiles, but we’d like more patients (to) take this to product status and use it as an actual treatment,” da Cruz says. Still, they are quite pleased with the system so far. “At the moment, we’ve got the cells, we’ve got the membrane, we’ve got the material, we’ve got the prototype of the injection system, and we have an operation— and the fastest we can do it is in 51 minutes, so it’s in the time frame we wanted and conceivably under local anesthetic,” he asserts. “So, we’ve achieved all the components. What we need to do now is make each of them optimal and that’s where the project is now.”
Other health issues
Although the USC system was designed for dry AMD and the London Project for wet AMD, both research groups believe they could potentially be adapted for broader vision problems.
“There are many cells that are damaged in different diseases, and we hope to use this as a paradigm for developing cells for different diseases and for more complex multicellular diseases (that would benefit from) delivery of more than one cell type,” da Cruz says. “That’s where we’d like to go.”
Humayun notes that the dry-AMD system could even reach beyond vision diseases. “The surgical tool obviously could help with other scaffolds that you need in tiny, tight spaces, but a big version of this could also help in orthopedic and cardiac applications,” he says.
Mostly though, Humayun hopes this new stem-cell-based system will help people who are struggling with vision loss from AMD. “At the end of the day, for me, it’s all about the patients we see. These are people who are otherwise very functional but they’ve lost their sight and it’s really led them to lose their independence. So, to be able to offer the Argus II artificial retina to individuals who are totally blind, or this RPE patch to those who are legally blind, it’s really very satisfying.” He adds, “Hopefully we can get this approved, as we did with the artificial retina, and begin helping these patients for whom, today, we really can’t do anything. That’s what we want to do.”
References
- K. L. Pennington and M. M. DeAngelis, “Epidemiology of age-related macular degeneration (AMD): Associations with cardiovascular disease phenotypes and lipid factors,” Eye Vis., vol. 3, no. 34, 20 pages, Dec. 2016.
- Second Sight, “Second Sight Medical Products receives FDA approval for Argus II System,” Feb. 2013. Accessed: Dec. 28, 2018. [Online].
- A. H. Kashani et al., “A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration,” Sci. Transl. Med., vol. 10, no. 435, p. eaao4097, Apr. 2018.
- L. da Cruz et al., “Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration,” Nature Biotechnol., vol. 36, no. 4, pp. 328–337, Apr. 2018.