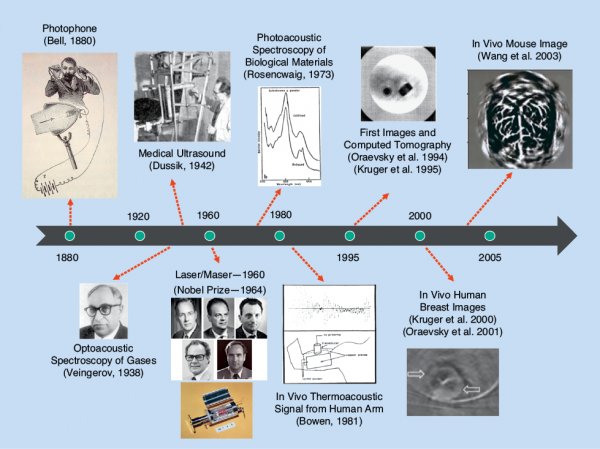
Despite the ancient discovery of the basic physical phenomenon underlying optoacoustic imaging and tomography [1], the lack of suitable laser sources, ultrasound detection technology, data acquisition, and processing capacities has long hindered the realization of efficient imaging devices. In fact, the first high-quality images from living animals were obtained about a decade ago (Figure 1), which was followed by an exponential growth of technical developments in instrumentation, algorithms, and biomedical applications surrounding this fascinating field. The ability of optoacoustics to probe optical contrast along a wide domain of penetration scales while maintaining excellent spatiotemporal resolution representative of ultrasound imaging, as shown in Figure 2, is unparalleled among the other optical imaging modalities [2], [3].
![The penetration depth and resolution of modern photonic imaging techniques. For living tissues, the methods at the left of the graph are primarily limited by light scattering, whereas the methods to the right are primarily limited by light attenuation in tissue, a parameter that depends on both absorption and scattering, or by ultrasound attenuation. Note that optical projection tomography (OPT) and selective plane illumination microscopy (SPIM) can operate deeper than the range shown in naturally transparent or chemically cleared samples. (2P/MP: two-photon/ multiphoton microscopy; DOT: diffuse optical tomography; FMT: fluorescence molecular tomography; MFT: mesoscopic fluorescence tomography.)(Figure adapted from [2].)](https://www.embs.org/wp-content/uploads/2015/05/mandal02-2409103.png)
State-of-the-art implementations of multispectral optoacoustic tomography (MSOT) are based on multiwavelength excitation of tissues to visualize specific molecules located deep within opaque living samples, which further allows simultaneous delivery of anatomical, functional, and molecular information from depths of several millimeters to centimeters in scattering tissues [4]. Thereby, MSOT has been widely employed for preclinical imaging, including in models of cancer, neurodegenerative and cardiovascular diseases, functional neuroimaging, kinetic and biodistribution studies, lymph node staging, and imaging of arthritis and inflammation. The main intrinsic tissue contrast at the visible and near-infrared wavelengths stems from the highly absorbent blood hemoglobin; however, other major tissue chromophores, such as melanin and fat, have also been shown to yield a spectroscopic optoacoustic contrast. Moreover, a large variety of photoabsorbing probes have been explored as contrast agents for attaining a specific extrinsic contrast. Altogether, these key advantages have prompted the development of high-throughput MSOT imaging systems for in vivo whole-body small animal imaging, further providing high sensitivity and spatial resolution, portability, and real-time operation capacity.
Small animal in vivo imaging is crucial in preclinical research to study changes in the organs at the cellular and molecular level, shedding light on the frontier of biomedical and pharmaceutical imaging research [2], [4], [5]. An ever-growing number of scientific articles are related to small animal imaging trials, and above all, MSOT is emerging as one of the most preferred noninvasive whole-body small animal imaging modalities for a number of key applications.
In this article, we outline the basic principles behind MSOT and trace the developments in the area of small animal imaging as well as their impact in preclinical research. The new realm of five-dimensional optoacoustic imaging [6], which consists of the three spatial dimensions, the time, and the spectral (optical wavelength) dimension, has revolutionized the modern functional and molecular imaging by using the synergistic combination of ultrasound- and light-related advantages, such as high spatiotemporal resolution and powerful spectrally enriched contrast. We further describe the experiences and challenges faced during the process of translating the technology from the lab bench into commercially available products.
Dimensionality in Optoacoustic Imaging
A large variety of approaches have been proposed for in vivo small animal optoacoustic imaging. Naturally, a single optoacoustic waveform represents one-dimensional information along the axis of the ultrasound detection element. Thus, two- or three-dimensional images can be rendered by raster scanning the detector in the two remaining spatial dimensions, as performed in acoustic or optical resolution optoacoustic microscopy [7]. Another technique consists in scanning an optical probe beam along a Febry-Pérot interferometric film to tomographically detect optoacoustically generated sound using an all-optical approach [8]. An alternative method for whole-body optoacoustic tomography was reported by Brecht et al., which was able to render the three-dimensional distribution of vasculature structures and blood-rich organs such as the liver, spleen, and kidney by rotating a matrix array transducer around the imaged mouse [9]. The real-time imaging capacity in whole-body observations was demonstrated by a cross-sectional MSOT system based on an array of cylindrically focused transducers [10]. This imaging geometry enabled capture of two-dimensional slices representing an entire cross section of living mouse at video rate (see Figure 3).
![(a) A schematic drawing of the cross-sectional MSOT system. A curved array of wideband and cylindrically focused ultrasound transducers enables parallel data acquisition. Optical fibers are used to homogeneously illuminate the object. (b)–(d) MSOT images of mouse anatomy taken at 750 nm. 1: kidneys; 2: spine; 3: spleen; 4: vena cava; 5: liver; and 6: brain. (Figure adapted in parts from [10].)](https://www.embs.org/wp-content/uploads/2015/05/mandal03-2409103-e1431631228338.png)
The capabilities of optoacoustic imaging were extended to four-dimensional imaging (three spatial dimensions + time) through the implementation of the spherical arrays of detectors [11]. Finally, the recently developed portable spherical array probe, combined with a fast wavelength tuning laser, real-time data acquisition, graphics processing unit (GPU)-based volumetric image rendering, and spectral unmixing, has enabled for the first time volumetric real-time spectrally enriched (five-dimensional) optoacoustic imaging at centimeter-scale depths [configuration shown in Figure 4(a)] [6], [12]. This portable system allows for convenient (handheld) handling of both preclinical experiments and clinical measurements in human subjects. The utility of the five-dimensional imaging approach can be further enhanced by tracking the kinetics and biodistribution of contrast agents with unique absorption spectra, such as the U.S. Food and Drug Administration-approved indocyanine green (ICG) dye that can be employed during in vivo studies to visualize vasculature, excretion through the liver, or retention in tumors [6], [10], [13]. More details on the mathematical methods supporting accurate optoacoustic image reconstruction are included in “Mathematical Methods in Multispectral Optoacoustic Tomography” [3], [14], [15].
![Dynamic contrast enhancement in nude mice with 4T1 tumors. (a) Single-pulse images obtained at 790 nm approximately 30 seconds after ICG injection and (b) multispectrally resolved ICG signal is overlaid in green. The spectrally resolved oxyhemoglobin (red) and deoxyhemoglobin (blue) within the tumor on days six (left) and 13 (right) demonstrate the label-free imaging capabilities of MSOT. (c) A photo of the cryoslicing through the tumor. The arrows indicate the hypoxic regions of the tumor core. (Figure adapted from [13].)](https://www.embs.org/wp-content/uploads/2015/05/mandal04-2409103-e1431631540932.png)
The Temporal Dimension
MSOT is based on the detection of acoustic signals created through the thermoelastic expansion of tissue under the influence of light, which is subject to three orders of magnitude less scattering per unit length in tissue as compared to ultrasound [4]. Thereby, the spatial resolution is significantly higher than that of diffuse optical imaging techniques. Imaging the distribution of light absorbers in three dimensions with high resolution is, however, not the only asset of MSOT. Indeed, other imaging dimensions may provide independent information regarding the imaged object.
Time represents a key dimension in imaging technologies, although it is often overshadowed by the spatial resolution performance of the modality as the latter is held responsible for “nice-looking images.” Yet, it is the high imaging speed that may enable artifact-free handheld imaging [6], [12], visualization of a beating heart [16], or real-time imaging of perfusion profiles in tumors [13] and the internal organs (e.g., the kidneys and brain) of small animals [10]. Fast imaging performance is greatly supported by the development of suitable algorithmic software capable of performing inversion and image/signal processing in real time, which is key for the successful implementation of four- and five-dimensional optoacoustic imaging. To this end, the use of GPUs has enabled visualization at frame rates of tens of volumes per second [6].
[accordion title=”Mathematical Methods in Multispectral Optoacoustic Tomography”]
MSOT allows the resolution of the biodistribution of chromophores having a specific optical absorption spectrum. For this, a two-step mathematical procedure is usually employed. On the one hand, a reconstruction algorithm is used to calculate the spatial distribution of the absorbed optical energy from the measured pressure signals. On the other hand, a spectral unmixing algorithm is applied to isolate the contribution of each chromophore from the measurements taken at multiple wavelengths.
As a tomographic approach, MSOT is based on the acquisition of signals at several locations surrounding the imaged object. The acoustic pressure at a given location can be expressed as
![]() (1)
(1)
with ![]() being the initial pressure, where
being the initial pressure, where ![]() is the (dimensionless) Grueneisen parameter,
is the (dimensionless) Grueneisen parameter, ![]() is the light fluence, and
is the light fluence, and ![]() is the optical absorption coefficient. For a constant Grueneisen parameter,
is the optical absorption coefficient. For a constant Grueneisen parameter, ![]() is proportional to the absorbed optical energy
is proportional to the absorbed optical energy ![]() . Optoacoustic. reconstruction is based on the mathematical inversion of (1), for which several approaches are applicable [15]. For example, (1), can be discretized to a matrix equation as
. Optoacoustic. reconstruction is based on the mathematical inversion of (1), for which several approaches are applicable [15]. For example, (1), can be discretized to a matrix equation as
![]() (2)
(2)
where ![]() is the model-matrix that maps the absorbed optical energy distribution
is the model-matrix that maps the absorbed optical energy distribution ![]() at the pixels (or voxels) of the region of interest to the pressure signals collected at a set of positions and instants
at the pixels (or voxels) of the region of interest to the pressure signals collected at a set of positions and instants ![]() . In a general case,
. In a general case, ![]() can incorporate acoustic propagation effects such as speed of sound variations or acoustic attenuation as well as the effects of the frequency response and shape (aperture) of the transducer employed to collect the signals. The distribution of the absorbed optical energy can then be estimated by numerical inversion of (2) as
can incorporate acoustic propagation effects such as speed of sound variations or acoustic attenuation as well as the effects of the frequency response and shape (aperture) of the transducer employed to collect the signals. The distribution of the absorbed optical energy can then be estimated by numerical inversion of (2) as
![]() (3)
(3)
where ![]() is a vector with the measured signals.
is a vector with the measured signals. ![]() is a suitable regularization operator, where
is a suitable regularization operator, where ![]() serves to weight the contributions of the data and regularization terms.
serves to weight the contributions of the data and regularization terms.
Multispectral imaging is based on tissue excitation at multiple optical wavelengths. For a given wavelength, the absorbed optical energy corresponds to a linear combination of the absorbed energy of all absorbing substances (chromophores) present in the
sample, i.e.,
![]() (4)
(4)
where ![]() and
and ![]() are the molar extinction coefficient and molar concentration of each chromophore. Spectral unmixing consists in estimating the spatial distribution of the concentrations of different chromophores from the optoacoustic images acquired at different wavelengths. This can be done, for instance, by expressing (4) in a matrix form as
are the molar extinction coefficient and molar concentration of each chromophore. Spectral unmixing consists in estimating the spatial distribution of the concentrations of different chromophores from the optoacoustic images acquired at different wavelengths. This can be done, for instance, by expressing (4) in a matrix form as
![]() (5)
(5)
where ![]() is the linear operator (matrix) relating the concentrations of the chromophores
is the linear operator (matrix) relating the concentrations of the chromophores ![]() to the optical absorption at different wavelengths
to the optical absorption at different wavelengths ![]() . As
. As ![]() is generally affected by local variations in the light fluence, accurate unmixing requires proper estimation of the light fluence distribution at the excitation wavelengths and inversion of (5) on a per-pixel (per-voxel) basis.
is generally affected by local variations in the light fluence, accurate unmixing requires proper estimation of the light fluence distribution at the excitation wavelengths and inversion of (5) on a per-pixel (per-voxel) basis.
[/accordion]
Optical Wavelength
Multispectral (or multicolor) imaging confers molecular specificity and, thus, provides the capability to quantitatively investigate biological conditions such as hypoxia and nutritional gradients as well as cell viability, proliferation, and drug response potentials. These parameters are essential in understanding the dynamics of living tissues and disease prognosis and progression. In multispectral imaging, different wavelengths are used for illuminating the tissue in a time-shared fashion. Fast-tuning optical parametric oscillator (OPO)-based lasers enable an entire multispectral scan to be performed in a subsecond time frame, where the wavelengths are chosen in a way to best sample the absorption spectrum characteristics of the specific chromophores and the contrast agent of interest. Spectral unmixing algorithms are then required to isolate the contributions of the individual chromophore(s) of interest to the optoacoustic signals representing the distribution of the total absorbed energy.
It is noteworthy to mention that blood offers a rich intrinsic contrast for label-free functional biological imaging as it is possible to distinguish between oxygenated (HbO2) and deoxygenated (Hb) hemoglobin (Figure 5). As an example of the five-dimensional optoacoustic imaging capabilities, Figure 5 displays the perfusion of brain vasculature in mice with ICG as a contrast agent. The wavelength dimension enables isolating contribution of the contrast agent through fast multispectral data acquisition and the subsequent reconstruction of agent distribution in real time. Yet, one inherent challenge in spectral unmixing is the so-called spectral coloring effect associated with the wavelength-dependent light attenuation [14]. The algorithms accounting for this effect are, thus, crucial to increase the accuracy and quantitativeness of the measurements.
![Five-dimensional imaging of mouse brain perfusion in vivo: (a) the layout of the experimental setup and (b) the single-wavelength images (maximal intensity projection along the depth direction) acquired before and after the injection of the ICG contrast agent. The results for two different concentrations are shown. When 10 nmol of ICG is injected, the contrast agent cannot be easily distinguished from the background blood absorption. Different structures in the mouse brain are indicated in the figure: the supraorbital veins (SV), inferior cerebral vein (iCV), superior sagittal sinus (SSS), confluence of sinuses (CS), and transverse sinus (tS). (c) A time series of images after spectral unmixing of multiwavelength data, taken for the 10-nmol experiment, clearly reveals the inflow of the agent in vivo and in real time. (d) The spectral excitation profile of several chromophores used for linear unmixing operations for identifying the molecular constitution of the tissue and the presence of the contrast medium. (Figure adapted in parts from [6].)](https://www.embs.org/wp-content/uploads/2015/05/mandal05-2409103-e1431632053232.png)
Technology Commercialization:
Opportunities and Demands
With its unique strengths as an imaging modality, MSOT has the potential to make a substantial impact on preclinical research as well as on the clinical market. Molecular imaging has been an important part of research and medicine for decades, but it has always come with limitations. For example, positron emission tomography (PET) has been extremely valuable in cancer diagnosis and pharmaceutical research. However, its use is limited by the use of ionizing radiation. Also, its spatial resolution is limited to 1–2 mm. MSOT, on the other hand, offers molecular specificity with at least ten times higher resolution without the safety concerns associated with radiation. In addition, it represents a low-cost alternative to PET and magnetic resonance imaging (MRI), where a substantial investment, infrastructure, and personnel are needed for operation.
In contrast to PET, MRI, or computed tomography (CT), clinical MSOT is portable, with a footprint and housing similar to clinical ultrasound. A clinician could, therefore, have access to molecular and functional information without the need for a specialized facility. There has also been a drive in the imaging community to combine molecular imaging modalities, such as PET or single-photon emission computed tomography, with the anatomy and function acquired with CT or MRI. These efforts have come at a substantial cost. Alternatively, MSOT combines anatomical, functional, and molecular imaging into a single modality, enabling a simultaneous readout of multiple metrics without expensive hybrid solutions or having to coregister sequential imaging datasets. And finally, MSOT holds promise for a multitude of clinical applications, including neonatal brain imaging, endoscopy, intravascular plaque assessment, intraoperative assessment of probe accumulation and/or hypoxia, dermatology, melanoma, sentinel lymph node imaging with ICG, and detection of breast cancer [17].
Despite its potential advantages over other imaging technologies, MSOT imaging faces challenges that are being actively addressed and researched. Multispectral measurements, for example, depend on sequential excitation with different wavelengths. It is, therefore, paramount that motion is minimized from the imaged object during the multispectral measurement. Reducing the time required for a multispectral measurement is one way of coping with such motion, and measurements at up to ten multispectral frames per second have been demonstrated. Increasing the speed of a multispectral measurement implies a corresponding increase in the repetition rate of the laser, which might impose limitations with respect to the maximal permissible exposure of the skin to laser light in clinical applications. An alternative approach consists of using two (or more) properly synchronized laser sources tuned to different
wavelengths [12].
One must, therefore, strike a balance between motion optimization, safety, and signal-to-noise ratio. The development of newer contrast agents as well as improvements in the spectral unmixing algorithms remain vibrant areas of research. ICG has been clinically approved since the 1960s; however, there are limited options for other molecules that absorb within the near infrared. And, finally, although regulatory-approved preclinical devices have already reached the market, medical device permissions in Europe and the United States are still pending. Once approved, multicenter trials in a number of key clinical applications will facilitate the transition of this technology from a highly potent research platform to an accepted medical device.
The Road Ahead—Hopes and Challenges
Optoacoustics has come a long way from its discovery to the present state-of-the-art small animal imaging scanners and experimental clinical handheld platforms. In less than a decade, researchers have been able to translate this technology from engineering laboratories into commercial products in preclinical imaging and further to translational medical imaging. Technologically, we have been able to demonstrate the rendering of spectrally resolved volumetric data in real time. State-of-the-art MSOT systems are able to accurately recover optical contrast at never-seen-before depths and speeds and, hence, offer promise in a range of biomedical applications both in research and in clinics.
Given the advancement of hardware capabilities, we are now able to exploit the data processing limits using high-speed GPUs and to import the realm of advanced inversion models, postprocessing, and machine learning to the modality. Parallel to the technical developments, innovations have taken place in areas of biomarker design and detection, leading to newer applications. The five-dimensional imaging capability thus enables researchers to visualize diverse endogenous chromophores and administered contrast agents. Currently, MSOT is being widely used in many research areas including in vivo cell tracking, molecular imaging studies, targeted molecular imaging, and functional imaging of the brain and heart. In spite of the vast progress, future work needs to be directed toward imaging at greater depths with enhanced accuracy and contrast.
References
- A. G. Bell, “Upon the production and reproduction of sound by light,” J. Soc. Telegraph Engineers, vol. 9, no. 34, pp. 404–426, 1880.
- V. Ntziachristos, “Going deeper than microscopy: The optical imaging frontier in biology,” Nature Methods, vol. 7, no. 8, pp. 603–614, 2010.
- P. Beard, “Biomedical photoacoustic imaging,” Interface Focus, vol. 1, no. 4, pp. 602–631, 2011.
- V. Ntziachristos and D. Razansky, “Molecular imaging by means of multispectral opto-acoustic tomography (MSOT),” Chem. Rev., vol. 110, no. 5, pp. 2783–2794, 2010.
- J. Xia and L. V. Wang, “Small-animal whole-body photoacoustic tomography: A review,” IEEE Trans. Biomed. Eng., vol. 61, no. 5, pp. 1–10, 2013
- X. L. Deán-Ben and D. Razansky, “Adding fifth dimension to optoacoustic imaging: Volumetric time-resolved spectrally enriched tomography,” Light: Sci. and Appl., vol. 3,
no. 1, p. e137, 2014. - L. V. Wang and S. Hu, “Photoacoustic tomography: In vivo imaging from organelles to organs,” Science, vol. 335, no. 6075, pp. 1458–1462, 2012.
- J. Laufer, P. Johnson, E. Zhang, B. Treeby, B. Cox, B. Pedley, and P. Beard, “In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy,” J. Biomed. Opt., vol. 17, no. 5, pp. 0560161–0560168, 2012.
- H. P. Brecht, R. Su, M. Fronheiser, S. A. Ermilov, A. Conjusteau, and A. A. Oraevsky, “Whole-body three-dimensional optoacoustic tomography system for small animals,” J. Biomed. Opt., vol. 14, no. 6, pp. 064007–064007, 2009.
- D. Razansky, A. Buehler, and V. Ntziachristos. “Volumetric real-time multispectral optoacoustic tomography of biomarkers,”
Nat. Protoc., vol. 6, no. 8, pp. 1121–1129, 2011. - A. Buehler, X. L. Dean-Ben, J. Claussen, V. Ntziachristos, and D. Razansky, “Three-dimensional optoacoustic tomography at video rate,” Opt. Exp., vol. 20, no. 20, pp. 22712–
22719, 2012. - X. L. Dean-Ben, E. Bay, and D. Razansky, “Functional optoacoustic imaging of moving objects using microsecond-delay acquisition of multispectral three-dimensional tomographic data,” Sci. Rep. vol. 4, article 5878, July, 2014.
- E. Herzog, A. Taruttis, N. Beziere, A. A. Lutich, D. Razansky, and V. Ntziachristos, “Optical imaging of cancer heterogeneity with multispectral optoacoustic tomography,” Radiology, vol. 263, no. 2, pp. 461–468, 2012.
- C. Lutzweiler and D. Razansky, “Optoacoustic imaging and tomography: Reconstruction approaches and outstanding challenges in image performance and quantification.” Sensors, vol. 13, no. 6, pp. 7345-7384, June 2013.
- A. Rosenthal, V. Ntziachristos, and D. Razansky, “Acoustic inversion in optoacoustic tomography: A review,” Current Med. Imaging Rev., vol. 9, no. 4, pp. 318–336, Nov. 2013.
- S. J. Ford, X. L. Deán-Ben, and D. Razansky, “Cardiac function and perfusion dynamics measured on a beat-by-beat basis in the live mouse using ultra-fast 4D optoacoustic imaging,” Proc. SPIE, vol. 9323, Mar. 2015.
- S. Zackrisson, S. M. W. Y. van de Ven, and S. S. Gambhir, “Light in and sound out: Emerging translational strategies for photoacoustic imaging,” Cancer Res., vol. 74, no. 4, pp. 979–1004, 2014.



