Sufferers of osteoarthritis are all too aware of the daily pain and impairment of swollen joints, of having to give up sports—and jobs—due to cartilage defects. What they may be less aware of is that three-dimensional (3-D) bioprinting and bioink technologies are being developed to revolutionize the existing (and often imperfect) treatments available to them.
“Articular cartilage has no capability to heal or repair itself, and few clinical approaches are capable of restoring long-term function to damaged or diseased cartilage,” explains bioengineer and regenerative medicine expert Tim Woodfield. The upshot is that osteoarthritis is a progressive joint disease currently treatable only by total joint replacement using permanent metallic and/or polymeric prostheses susceptible to long-term wear and loosening. Woodfield’s innovations with bioinks have the potential to change that.
Working at the interface of stem-cell biology, biomaterials science, and orthopedic surgery, regenerative medicine researchers like Woodfield are pursuing living tissue substitutes to repair or regenerate damaged or diseased tissues. The demand for these solutions is significant. In New Zealand, where he is based, Woodfield says, “osteoarthritis is the most common cartilage-related disease … [and is] more prevalent than asthma … mental disorders, diabetes, and cancer.” With New Zealand’s elderly population living longer and more active lives, the spread of degenerative joint disease is, as Woodfield puts it, an “epidemic” [2].
The need is not confined to New Zealand. “The global market for cell therapy and regenerative medicine products,” he continues, “is expected to be US$39 billion by 2021 [3], with the largest segment (60%) comprising orthopedic/musculoskeletal applications (e.g., the cartilage, bone, and tendon).” The demand for total joint replacement and revision surgery, he adds, “will increase 250% in New Zealand by 2030 [4] and skyrocket by 673% in the United States during the same period.” [5]

An associate professor at the University of Otago, Christchurch, Woodfield leads the Christchurch Regenerative Medicine and Tissue Engineering (CReaTE) Group (Figure 1). They are pioneering forms of bioink and 3-D bioprinting that offer tissue engineers the advantage of controlling the architecture, or arrangement, of the printed cells, allowing them to more closely mimic the native tissue in their tissue constructs.

Woodfield’s research is not an isolated offshoot in New Zealand. The country is home to a growing nexus of research labs, clinicians, medical device companies, and a government-sponsored Consortium of Medical Technology driving advances in regenerative medicine and the technology that supports it. Among other headliners in the field is Prof. Jill Cornish (Figure 2, right), a biologist leading the Cell and Molecular Bone/Joint Biology Research Laboratory at the University of Auckland. Cornish and Woodfield are frequent collaborators; together, they are the principal investigators for one of New Zealand’s Center of Research Excellence teams. Their two labs are multidisciplinary hubs where cell biologists, orthopedic surgeons, and engineers routinely work together with the goal of preserving joint function and quality of life for those with musculoskeletal injuries or disease. The variety of roles needed in the solution design reflects the complexity of the tissues they’re attempting to construct.
Rotator Cuff Tears—and Retears
Cornish, who has spent decades scrutinizing healing and regrowth at the cellular level, is deeply familiar with the body’s inherent capability to heal itself. “Bone is a beautiful repairer in itself,” she says. “The host has, within [its] tissue, cells [that] are capable of replacing the old ones. Bone is continually turning over right throughout life. Your entire skeleton is replaced every ten or so years, whether you’re a baby or you’re in your 100s.”
Cornish is equally aware of scenarios when this remarkable self-repair comes up short—when damaged tissue, bone, or cartilage cannot return itself to its preinjury state. Consider a common shoulder injury in people over 50: rotator cuff tears. “There is a huge burden with the rotator cuff throughout the world that’s generally [occurring at a rate] of over 50% in over-50-year-olds, and they have significant pain and functional limitations,” notes Cornish. “And there’s very, very poor healing,” she adds, if a person leaves the injury to heal on its own without surgery. Even with surgery, the likelihood of another tear occurring afterward is discouraging: retear rates are as high as between 41 and 69%, depending on the type of surgical repair and size of the tear [1].
Often, the repeat tear is precipitated by a person’s seemingly innocuous movement in attempting to regain everyday mobility. A patient five weeks postoperation might do a few minutes of raking in his or her backyard, only to be hit 20 minutes later with an alarming shoulder pain. The patient won’t know until after another six to eight weeks of physical therapy if that pain was the tendon tearing again. In many cases, a second surgery isn’t likely to succeed due to the shredding of the tendon and the lack of healthy tissue. Such a bleak prognosis is, unfortunately, not uncommon and only likely to proliferate as the world’s older population continues to grow.
Engineering a Tendon Scaffold
But what if medicine could intervene differently in such cases? What if orthopedic surgeons could do more than patch up damaged tissue or stabilize bone with metal implants? What if they could also—or alternately—introduce a biological boost into the tissue, amplifying its inherent ability to regrow, in cases where the body has fallen short of being able to fix itself?
Cornish’s lab concentrates on regenerative medicine with the goal of doing just that. In the case of torn rotator cuff tendons, the group is refining an implant that would provide mechanical support for the damaged tendon (the traditional role of implants), while also supporting its ability to regenerate and strengthen so that it won’t tear again. More specifically, Cornish and her team are on the hunt to find just the right combination of materials to form a scaffold for the torn tendon.
Reproducing the existing procedures of rotator cuff surgery, the team uses “the same sutures and the same materials to wrap the tendon and hook it back into the bone,” Cornish explains. “But at the same time, we add a patch.” This “patch” (the scaffold) is made from collagen-based materials and lies alongside the patient’s tendon cells. True to its name, the implant is designed to be an organizing construct for the cells, similar to the way an architectural scaffold supports the construction of a new building (Figure 3). The engineered tissue scaffold promotes cell growth to maintain or bring in a blood supply that may have been ruptured when the injury occurred.
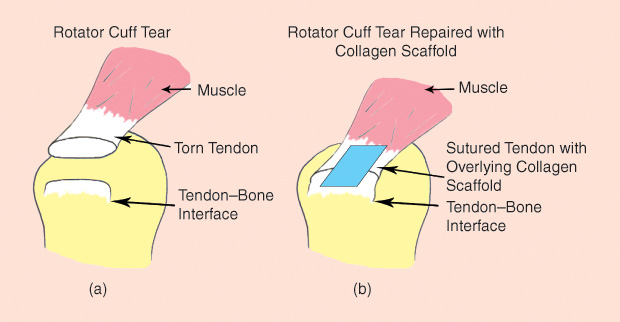
Other approaches in tissue engineering have focused on adding stem cells to a musculoskeletal defect to boost new tissue growth. Cornish’s lab has sought instead to identify and test different skeletal bioactive factors (natural or synthetic) that can be injected into a scaffold to prompt growth in the host’s own tissue, which could prove to be a more scalable solution than using stem cells.
Identifying Biomaterials That can Thrive in Vivo—and Stretch
Tissue engineering’s early successes in the 1980s and 1990s in engineering simpler tissues (i.e., those that are relatively avascular with a low metabolic rate) led many to think that the same methods would work with more complex tissues—that labs would be making hearts in a dish within a decade. Those predictions did not come to pass. Researchers (and startup investors) learned subsequently that it was not just a matter of setting any cell type in a matrix, implanting that construct in a human, and then watching it grow. Complex tissues and large organs have proven much more difficult to engineer. Today, the field’s biggest hurdles center around scalability (for clinical applications), reliability of the cell sources and biomaterials used, and host integration (designing implants that don’t get rejected by the host’s immune system).
The latter challenge of immunoreceptivity is the current focal point for the rotator cuff patches Cornish’s team is developing. To date, there’s no ideal implant. “It’s close, but we’re not there yet,” Cornish says, regarding the in vivo trials which have inserted the patch implants in human shoulders. “Some have failed because they have been caught in a foreign body response. Initially they are good, and then about six weeks later … the host’s immune system starts to reject the scaffold.” These rejections, she explains, are due to insufficient in vitro evaluations.
In 2015, Cornish’s group had some success in testing a new biomaterial scaffold made from ovine forestomach extracellular matrix (OFM) in rats. They found the OFM would support tenocyte growth—with no in vitro immunogenic response. Tendons augmented with the OFM scaffold “improved the quality of the healing,” says Cornish, but there was no evidence of biomechanical advantage for the tendon. In other words, there was no evidence that the OFM scaffold could help repair a tendon to the point that it wouldn’t tear again.
“That’s one of the reasons we’ve set up this package of evaluation of the scaffolds,” she says, referring to a process her lab has designed to put their constructs through rigorous mechanical tests in a “cell gym.” It’s an evocative name, suggestive of cells doing burpees or lifting barbells. As Cornish explains, the cell gym allows her team to “add the cells on the scaffold in a piece of apparatus so that the actual scaffold and the cells on it can be stretched to mimic what is happening in the animal.” In other words, tests that ultimately aim to assess whether the human (with the implanted engineered tissue) will eventually be able to do burpees—or at least lift a heavy purse without pain.
Getting Cells to Come Together, Layer by Layer
On New Zealand’s south island, Tim Woodfield’s CReaTE Group is innovating methods of producing biomaterials for orthopedic use, primarily for cartilage and bone regeneration. As with the tendon cells Cornish needs to stretch to mimic real-use cases in the host, engineering cells to repair cartilage and bone (both structural tissues) means creating implants that, ultimately, are capable of performing a job in the body—that can “regenerate damaged or diseased tissue and at the same time allow us to put load or weight on our joints and to move pain free,” as Woodfield puts it.
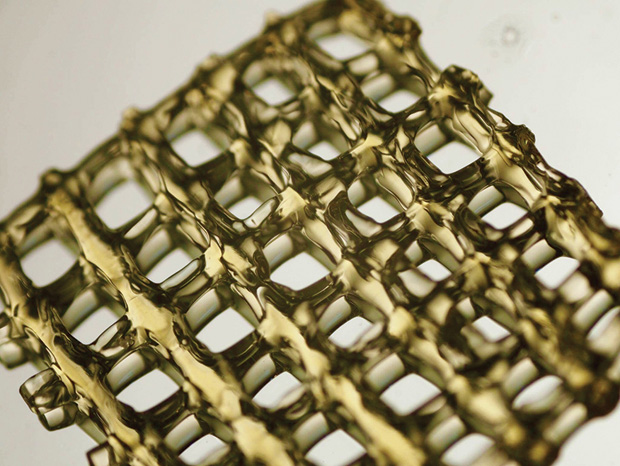
His group is working on a modular 3-D bioprinting approach they call “tissue assembly,” that builds small modules of tissues in layers (Figure 4). Imagine LEGOs being stacked to create a larger shape—only these cellular building blocks are spherical, rather than rectangular: little balls made of cells or cells combined with bioink materials (Figure 5).
Contrast CReaTE’s modular approach with the more common method in tissue engineering: setting up a porous scaffold to which cells will stick. Porous scaffolds entail obtaining a large number of cells individually and then hoping they will thrive, filling in the gaps of the scaffold, to eventually repair the damaged tissue. “While that’s been partially successful,” says Woodfield, “it hasn’t been a great solution. It doesn’t really put the cells in the best possible environment that will make them as ‘happy’ as they need to be to make a large amount of tissue.” Individual cells joined together via a porous scaffold often don’t perform over the long term because the process results in a random arrangement of cells.
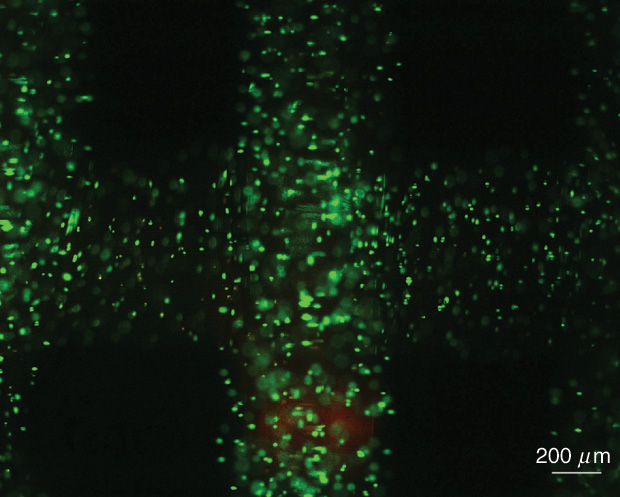
“The main advantage of bioprinting is that you can put the cells where you need them,” explains Woodfield. The challenge is “learning the clues from cell or stem cell biology to know how best they should be arranged and grow.”
“Just having cells doesn’t solve the problem,” Woodfield emphasizes. It’s imperative to 3-D print or assemble engineered cartilage tissue with the same organization and structure as normal cartilage so that the right type of tissue is being made—and sustained. For example, the goal is to get blood vessels to form at the same time as the bone tissue cells, so they can deliver the nutrients necessary to sustain the new tissue.
If the structure’s wrong, the implanted tissue will wear out over time … or die. Well-engineered living bone or cartilage tissue will be able to perform structural tasks—allowing joints to move freely and without pain—without the ten- or 15- year expiration date placed on many metal hip and knee joint replacements of today.
Bioprinting with Bioinks
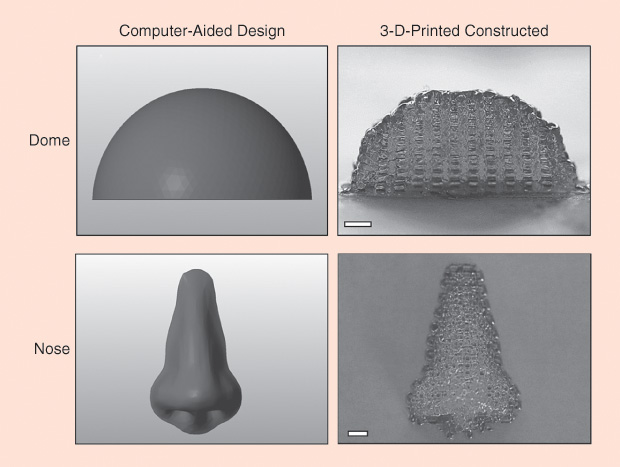
The viability of tissue assembly is propelled by Woodfield’s successes with bioink, the foundational ingredient for bioengineers using a 3-D bioprinter to print constructs containing cells (Figure 6). Woodfield’s research indicates that tissues assembled from bioinks are much better than the porous scaffolds at reproducing the complex arrangements of cells in the body. Tissue assembly also addresses the challenges of cell sourcing and scalability—the ability to engineer larger pieces of tissue than most labs are able to produce now, so that they can be used in patients who have significant bone or cartilage defects such as osteoarthritis.
Woodfield has been experimenting with bioprinting since 3-D printing technology emerged in the early 2000s, when the process was anything but straightforward. His initial success in bioprinting scaffolds exhibited methods for gaining more control over the process (other bioprinted tissue results had tended to be more random) and achieving a higher quality of engineered tissue.
As more bioengineers turned to 3-D printing, a period of rapid development of the hardware and technology ensued, with materials-specific machines being created to print metal, ceramics, or cellular constructs. At the same time, less attention was being given to the bioinks themselves. “Five years ago,” Woodfield recalls, “everyone was working with just one or two types of materials.” That has since changed.
The last few years have seen a surge in bioink development. Research has found gelatin hydrogel materials to be, as Woodfield puts it, “quite a nice environment for cells or stem cells to be caked in.” When heated to cell-friendly temperatures such as 37 °C, a gel-based bioink can flow out of a nozzle. Then it cools rapidly, setting into a solid. “You don’t want to print a puddle,” he points out. To properly set the printed construct, most researchers use ultraviolet (UV) light to activate the cross-linking that polymerizes the gel—not unlike the way adhesives interlock to solidify their bonding. But UV light, as we know, can damage cells, and researchers have been looking for alternative ways to cross-link the gel.
In 2016, Khoon Lim (pictured above in Figure 1), a research fellow in Woodfield’s lab, made a breakthrough. Lim had prior experience using visible light for polymerization of materials other than bioinks. In Woodfield’s lab, he applied what he knew of visible light chemistry to cross-link the bioinks. It worked. Visible light was not only gentler on stem cells and cartilage cells than the UV light, as expected, but also was better at cross-linking. Currently, Woodfield’s group is about to publish the results of other work they’ve done in bioprinting extremely intricate, very-high-resolution features using this visible-light processing technique.
Woodfield believes that further success in forming increasingly viable tissue constructs lies in improving the material’s chemistry. “The opportunity is less about just working with a 3-D printer,” he clarifies. “I think a lot of people perceive that if you’re 3-D printing it, then it’s got to be better. It isn’t necessarily that way. … [Bioprinters] give you a very advanced and exciting tool to have much more control over what it is that you can manufacture … [but] you still have to take it in combination with whatever type of material you’re trying to work with.”
Time to Arrival
Even with increasingly cell-friendly bioinks, Woodfield predicts it could still be another ten to 15 years before the 3-D tissues printed from them make it into patient’s joints. Currently, the small 3-D tissue models his lab produces can be used for drug screening, but they are not yet at the stage of clinical trials.
“Engineering tissues, even if the tissues appear to be quite simple, is a very, very difficult process,” he says. “The perception of the global tissue-engineering community was that by 2005, we would have solutions, and we essentially still don’t. It is a long-term game,” he admits. Gone are the overreaching predictions for tissue engineering in its early days. Today, practitioners are grounded in the complexity of testing and refining the manufacture of healthy tissue that will survive the exigencies of movement— and acceptance—in a human body.
Cornish’s recent findings regarding lactoferrin’s ability to boost bone growth are a perfect example of the multistage problem solving entailed in testing even a single bioactive factor. Lactoferrin is a large glycoprotein molecule found in the milk of humans and cows. It is also an “extremely potent and safe bioactive factor for increasing bone growth” of broken bones, Cornish explains. Her lab has succeeded in triggering “astounding” bone growth in preclinical models of bone defects, but they have not yet found the right scaffold to release the lactoferrin at a slow enough rate (Figure 7). “We put it into a gel, and it seeps out over a period of about six hours and then it’s gone,” she says. While this six-hour treatment of the host bone is able to set up a reaction that promotes bone growth for 12 to 16 weeks, a slower release rate could be even more effective in aiding bone regeneration.
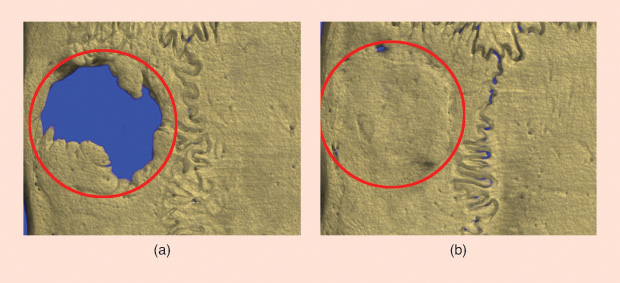
Still, there can be a lack of understanding from the public about the time it takes to land on a clinical solution. People may hear, for instance, of the amazing effect of lactoferrin on bone growth and wonder, “Well, why aren’t you putting that into my mom’s hip now?”
If all biologists threw things into bodies that appeared to be good on the tissue plate,” Cornish points out, “then we’d have immense problems.” That said, “There’s a number of people around the world trying to look into this right now. … There will be a scaffold,” she promises.
Despite the fact that researchers like Woodfield and Cornish have signed on to a long game, the aim of seeing clinical applications for his work motivates Woodfield every day. As he says, “We want to be able to hand surgeons their next iteration, the next generation of some clinical solution [that] is actually going to help patients”—whether they’re humans, horses, or dogs (his lab also works on veterinary solutions).
For the nearer future, the CReaTE Group has received a NZ$3 million grant from the New Zealand government’s Ministry of Business, Innovation, and Employment to work with industry partners Enztec and Ossis in developing the next generation of 3-D-printed titanium joint replacements, which allow patients’ bones to grow through and around the implants. By encouraging bone growth between the implant and the host bone, the researchers believe they can improve on existing treatments for many orthopedic complications and failures.
And even when the regenerative tissue implants of the future arrive, Woodfield predicts they won’t displace the old metal implants overnight. Instead, it’s likely to be a natural progression, either in the form of hybrid constructs being phased in or as an overlap phase in which titanium implants decrease while more cell-based degradable solutions are applied. This measured, gradual vision for an orthopedic revolution is of a piece with the care taken in the field. In Woodfield’s view, it’s important to “make sure that we get the biology right.”
References
- M. Street, A. Thambyah, M. Dray, S. Amirapu, D. Tuari, K. E. Callon, J. D. McIntosh, K. Burkert, P. R. Dunbar, B. Coleman, J. Cornish, and D. S. Musson, “Augmentation with an ovine forestomach matrix scaffold improves histological outcomes of rotator cuff repair in a rat model,” J. Orthopaedic Surg. Res., vol. 10, p. 165, Oct. 2015.
- Access Economics. (2010, Apr. 13). “The Economic Cost of Arthritis in New Zealand in 2010.” [Online].
- Markets and Markets, Regenerative Medicine Market Report— Forecast to 2021. Report BT4419, 2017. [Online].
- Hooper G. “The ageing population and the increasing demand for joint replacement,” NZ Med. J., vol. 126, no. 1377, pp. 5–6, 2013.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. “Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030,” J. Bone and Joint Surg., vol. 86, no. 4, pp. 780–785, 2007.



