Cell phone chimes, sticky notes, even the proverbial string around a finger—these timehonored external cues help guard against our inevitable memory lapses. But some internal help to the brain itself may be on the way in the form of what’s being called memory prosthetics. Once considered to be on the fringes of neuroscience, the idea of adding hardware to the brain to help with memory has gathered steam. In 2014, the U.S. Defense Advanced Research Projects Agency (DARPA) made a US$30 million investment in memory prosthetic research as part of the Obama administration’s Brain Research through Advancing Innovative Neurotechnologies initiative. In August 2016, Kernel, a startup based in Los Angeles, California, announced its goal to develop a clinical memory device for those debilitated by neurodegenerative disorders such as Alzheimer’s disease.

“We are trying to think about new neurotechnologies that could be used to help individuals form and recall memories again,” says Justin Sanchez (Figure 1, right), program director of DARPA’s Restoring Active Memory (RAM) project. The project was motivated by the numbers of military personnel who experience memory impairments associated with traumatic brain injury (TBI). The plan is to demonstrate by 2018 that the implantable device can restore the ability to form and recall memories in human clinical populations.
In fact, the age of the bionic brain has already arrived. Other neural prosthetics allow paralyzed people to move robotic limbs, deaf people to hear through cochlear implants, and those with epilepsy to avoid seizures. In each case, the device interacts directly with the brain, substituting or compensating for a damaged component. Researchers hope to apply this to memory circuitry, which degrades precipitously in Alzheimer’s disease or can be damaged by TBI, making it difficult for people to take care of themselves and straining both families and the healthcare system. The number of individuals living with severe memory impairments is growing; 270,000 people in the military have been diagnosed with TBI since 2000, and Alzheimer’s disease diagnoses are projected to reach 13.8 million by 2050.
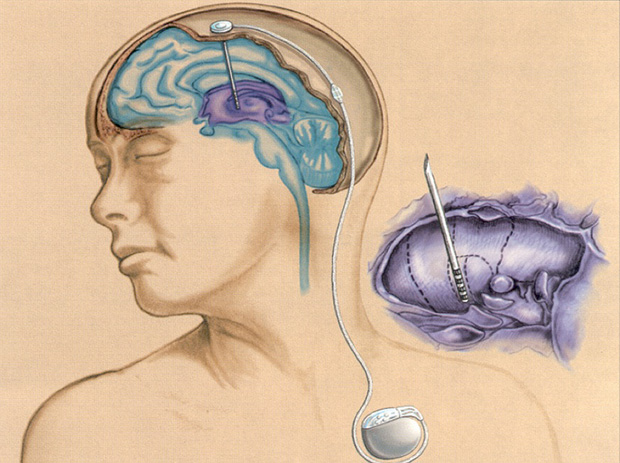
Yet, within a decade, hardwired help may be on the way. Engineers, neurologists, and neuroscientists have teamed up to develop memory prosthetics that precisely inject electrical current to shore up memory circuits. A clinical trial of people with mild Alzheimer’s disease has found that deep brain stimulation (DBS) of one brain region can keep memory circuitry online (Figure 2). In another approach, two DARPA-funded projects aim to strengthen memory by firming up the initial formation, or encoding, of a memory. This involves a device that can sense when the ongoing chatter of neurons is less than necessary to encode a memory and then bump up neural activity accordingly. Such devices consist of tabletop versions now, but researchers are striving to make them fully implantable. The efforts are pushing the limits of understanding human memory and electronics alike.
“There’s virtually no part of the memory circuit that we’re not investigating here,” Sanchez says. “That’s important because, when it comes to human memory, we don’t know all of the specific circuits that are involved in memory formation and recall, so we’re learning about human memory while we’re developing the prosthetics for restoring memory.” Memory prosthetics could target ailing circuitry with more precision than drugs. “A pharmaceutical pretty much bathes the brain and the body in the chemical,” Sanchez continues. “That’s why the device approach, which would allow us to directly interact with the specific neurons involved in memory formation and recall, is a direction we aim to go.”
Keeping Memory Networks Online
A memory is formed by a network of brain regions centered around the hippocampus. Experiences, such as meeting a new person, result in patterned electrical activity in the hippocampus, which then sends pulses to other parts of the brain to store a memory. When we see that person again a few days later, the circuit kicks in to retrieve the memory and deliver recognition. But the involvement of multiple brain regions, as well as precise patterns of activity, makes it difficult to know how best to tinker with the electrical messages passed around by this network in a way that shores up memory.
Nevertheless, there are signs that it can be done. In 2012, researchers at the University of California, Los Angeles, reported that DBS to the entorhinal cortex, a region that plugs information into the hippocampus, could enhance memory in humans [1]. The researchers studied seven people with intractable epilepsy, all of whom had received temporary implants of electrodes into their brains, to help doctors identify the seizure-prone regions that needed to be surgically removed. This required monitoring brain activity for many days, during which researchers could study memory while the patients navigated a virtual environment. If milliamp stimulation was applied when a patient encountered a particular virtual location for the first time, in later trials the patient could find that location more quickly and accurately.

This demonstrated gains in normal memory, but Andres Lozano (Figure 3, right), a neurosurgeon at the University of Toronto in Canada, has begun to explore DBS as a way to save brain function in people with mild Alzheimer’s disease. Although an electrode implanted into the brain might seem drastic at the early stages of illness, it’s worth the risk to some. “The average survival after an Alzheimer’s diagnosis is on the order of six to nine years, and you will die of your dementia,” Lozano says. “Patients who do not accept that are the ones that are the most keen to undergo this investigational procedure.”
Lozano and his colleagues have shown that as Alzheimer’s disease progresses, different parts of the brain shut down in a characteristic sequence. In a small phase 2 clinical trial, Lozano found that applying DBS to the fornix, which conveys signals coming out of the hippocampus, could revive dormant parts of the brain. After a year of continuous stimulation, no adverse effects were reported, but no clear sign of memory enhancement was seen either. “If we get these brain areas to turn back on again, that might mean that their function could return, and that might lead to improvement in Alzheimer’s disease,” Lozano explains, noting that a phase 3 trial is being planned.
Computing Memory
Another way to boost memory is to create stronger memories in the first place. To explore this possibility, two separate DARPA-funded research groups participating in the RAM project are developing devices that both read out ongoing brain activity and write in signals as needed. This requires electrodes to monitor neural activity, a microprocessor to analyze these signals, and a stimulator to send current back through the electrodes.
One group’s device is meant to mimic the function of the hippocampus itself. “We’ve tapped into the hippocampus because we think that almost anything that needs to be remembered will be represented in that structure in some way,” says Sam Deadwyler, a neuroscientist at Wake Forest Baptist Medical Center in Winston-Salem, North Carolina. Deadwyler and Robert Hampson (Figure 4), also at Wake Forest, have teamed up with Theodore Berger and Dong Song at the University of Southern California, Los Angeles, to develop their device. Their research stems from experiments begun more than 20 years ago, which revealed that certain patterns of hippocampal activity marked strong versus weak encoding states when rats did a memory task.

Specifically, rats were presented with a lever that they pressed to receive a reward (the encoding phase). The lever then retracted, and, after a delay, two levers emerged. The rat then had to choose the lever that it had not pressed, thus retrieving its memory. The researchers realized that during encoding, activity in the hippocampus reflected whether the rat would later remember the lever during the retrieval phase. “We could identify what we called strong codes and weak codes. Strong codes would result in correct performance, weak codes would result in errors,” Hampson says. “Then our question became, ‘could we make weak codes strong?’”
They teamed up with Berger and Song, who had developed a multiple-input, multiple-output (MIMO) computer model of information processing in the hippocampus. This MIMO algorithm could take in information from many different neurons and predict their effect on the neurons receiving the information. For Deadwyler and Hampson, this meant that the algorithm could translate a message passed between the CA3 and CA1 subregions of the hippocampus, thus allowing their device to deliver the needed electrical signals to produce strong encoding in CA1. In fact, introducing stimulation when the MIMO model detected weak encoding improved rat performance under normal conditions and when hippocampus function was hobbled pharmaceutically [2]. Because the device is meant to embody hippocampal computations, it should work for anything that the hippocampus might process, no matter the event or task. New work in monkeys supports this: stimulation during weak encoding aided memory, whether for an object or its location [3].
Deadwyler and Hampson foresee a clinical trial of their memory prosthetic in five to ten years, and they have begun experiments in people with epilepsy awaiting surgery. Researchers consider such memory prosthetics to be as safe as DBS electrodes, which have been used for 30 years. The currents delivered to the brain are well below levels that can cause damage. Furthermore, because the device is meant to stand in for the hippocampus, it is unlikely to encode unnecessary information, Hampson says. “Our goal with the neural prosthetic is not to input specific information code patterns to the brain, but rather to create a device which ‘bypasses’ a damaged hippocampus by performing the same processing functions that an intact hippocampus would perform in the same context.”
Memory Mapping

The second RAM project research group, run by Michael Kahana at the University of Pennsylvania in Philadelphia, is also studying memory in an epilepsy patient population, but with consideration of the contributions of additional brain regions. Because electrodes are placed in the brain based on where doctors believe the seizures to originate, they sample beyond the known memory network. This approach has allowed unprecedented access to the machinations of the human brain: in two years, the group has enrolled more than 200 people with implanted electrodes, conducted upward of 1,000 memory-mapping sessions, and collected data from more than 20,000 electrode contacts. The research builds on the work of Kahana and Daniel Rizzuto (Figure 5, right), also at the University of Pennsylvania, who have identified neural signatures of good memory performance in humans. Their device will use stimulation to encourage an optimal brain state for forming memories—similar to being “in the zone” during our daily lives, Rizzuto says.
For example, in a memory task called a delayed-recall task, a person studies a list of words and is then asked to recall those words minutes later. For the RAM project, the researchers have trained a machine-learning classifier to interpret patterns of brain activity while a person studies—that is, encodes—the word list. Preliminary results suggest that the classifier can predict with some accuracy whether or not a word will be remembered. Stimulation can also modulate memory, for better or worse. “If stimulation arrives when the brain is working well, it tends to impair performance,” Rizzuto says. “If the brain is in a poor state, stimulation tends to boost performance. This tells us that timing of stimulation is critically important” (Figure 6).
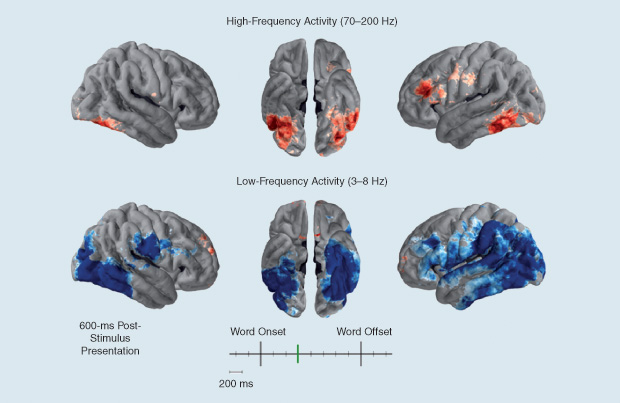
Researchers are beginning to do closed-loop experiments in which stimulation is delivered as needed, when the classifier deems encoding to be weak. This means data from the brain need to be read out and signals sent back into the brain within around 100 milliseconds, according to Rizzuto.
Ultimately, DARPA wants a fully implantable, wireless device, but, for now, the RAM projects are working with tabletop versions. For example, the hardware developed by Rizzuto’s group fills a rack that must be wheeled in next to the patient’s bedside and plugged into the wires coming from the patient’s brain. But miniaturization is on the horizon: their next-generation device is the size of a stack of five iPhones. Eventually, the device could be implanted underneath the skull and powered through the skin, maybe through a cap or a pillow. Improvements in the electrodes may also give finer resolution of brain activity and more informative signals.
The required electronics to collect high-fidelity signals from thousands to millions of neurons, amplify and interpret them, and then send precise stimulation back to the neurons—all in a small, portable package—don’t necessarily exist yet. “There’s a huge list of technological developments that needs to be accomplished in order to make this work,” Sanchez says. Whether insights from these memory-mapping sessions will prove helpful to a memory-impaired person remains to be seen. But, as Rizzuto notes, “We are optimistic that the tasks we’re using will translate very well to the clinical population in a more ecologically valid type of setting, like when they’re at home trying to remember what’s on the grocery list or where they are going.”
References
- N. Suthana, A. Haneef, J. Stern, R. Mukamel, E. Behnke, B. Knowlton, and I. Fried, “Memory enhancement and deep-brain stimulation of the entorhinal area,” N. Engl. J. Med., vol. 366, pp. 502–510, Feb. 2012.
- T. W. Berger, R. E. Hampson, D. Song, A. Goonawardena, V. Z. Marmarelis, and S. A. Deadwyler, “A cortical neural prosthesis for restoring and enhancing memory,” J. Neural Eng., vol. 8, no. 4, Aug. 2011.
- S. A. Deadwyler, R. E. Hampson, D. Song, I. Opris, G. A. Gerhardt, V. Z. Marmarelis, and T. W. Berger, “A cognitive prosthesis for memory facilitation by closed-loop functional ensemble stimulation of hippocampal neurons in primate brain,” Exp. Neurol., to be published.



