For many therapeutic interventions, imaging has become an essential component of initial diagnosis, as well as the monitoring of treatment progress and outcome. Imaging data can now be acquired in real-time, which has led to the emergence of image-guided interventions where therapies are guided by intra-procedural imaging (e.g., ultrasound, CT, MRI). Examples include catheter-based therapies such as stent placement, or chemo-embolization where chemotherapy plus embolizing agents are infused at high concentration directly into the tumor through catheters placed in tumor feeding vessels. Other examples include radiation therapy, various cardiac interventions [1], therapeutic ultrasound, or localized thermal therapies.
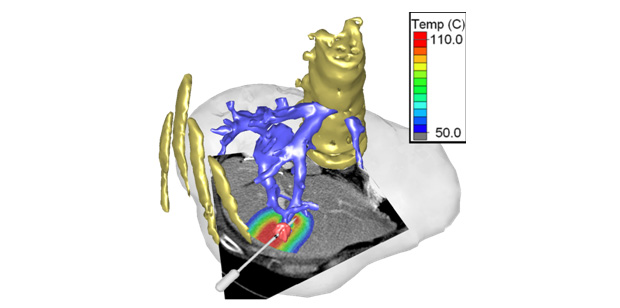
The readily available pre-procedural medical image data can also be employed for patient-specific simulation of therapeutic interventions based on computational models. Particularly for complex interventions where the best approach is not obvious, computer simulations could predict outcome of a specific intervention, allow interactive exploration of different options, or suggest an optimal approach to the treating physician. In a few select cases this is already reality; for example, in intensity-modulated radiation therapy, patient-specific computer simulations are widely used to predict spatial patterns of radiation exposure, in order to devise a treatment plan that achieves optimal radiation dose delivery to the tumor while limiting exposure to non-targeted tissues. There are many other areas where similar treatment planning platforms may be of value, including those listed above or thermal cancer therapies (Figure 1).
In addition to treatment planning of image-guided therapies, these platforms could be adapted as training software. Such a training platform would allow a trainee (e.g., medical fellow) to perform a certain procedure in virtual reality without risk to a patient. In other clinical specialties (e.g., surgery), training software has been shown to accelerate learning of complex procedures and improve procedure outcomes, and similar benefits are likely for interventional procedures.
However, several challenges need to be addressed. First, based on the imaging data of a specific patient, anatomical structures need to be identified (termed “image segmentation”) to create a computer model geometry for a specific patient (see Figure 1). In addition, spatially varying tissue parameters may be required based on additional imaging data (e.g., perfusion maps); the therapeutic device must be modeled and placed at, or within, the patient-specific model; and depending on the mathematical modeling approach, the geometry may need to be divided into small elements (termed “meshing”). Also, the biophysics corresponding to the specific therapy has to be simulated; this can include modeling of biomechanics (e.g., prosthesis), fluid dynamics (e.g., stents, heart valves), electromagnetics (e.g., heart or muscle stimulation), ultrasound wave propagation (therapeutic ultrasound), heat transfer (e.g., thermal ablation or hyperthermia), etc. For real-time use, the model results must be available within an adequate time frame, which may require algorithm optimization (e.g., GPU based acceleration methods). Finally, model results must be appropriately visualized for the treating physician, the system must be integrated into the clinical workflow, and the computer simulation must be validated, which ultimately means comparing predicted to actual outcomes in treated patients.
Many of these challenges currently still involve considerable user interaction or computational time. For example, both the identification of various structures from anatomical images (segmentation), as well as meshing, are often too time consuming for clinical use since they require significant user interaction. Often, the simulation time is still on the order of hours for a 3D simulation, inadequate for use in an interactive platform. Finally, adequate validation is in many cases missing.
While it is likely that in the coming years patient-specific computer models will become available to assist in treatment planning of complex procedures, we are still in the early stages of research and adoption of such systems. Any clinical translation of such platforms will ultimately depend on adequate validation, seamless integration into clinical workflows, and demonstration of improved outcomes and/or safety of the procedures.
References
- Muller, J., “Inside a Beating Silicon Heart,” Forbes, Jan. 6, 2014.


