The increase in diabetes is a major threat to health and economic development in the 21st century, as stated by the United Nation’s Millennium Development Goals. In 2013, 380 million people worldwide had diabetes, and almost 600 million are expected to have it by 2035. Diabetes is the major cause of nontraumatic amputation and blindness in developed countries. It is also an expensive disease. In the United States, one in five dollars for health care is spent on diabetes. Net losses in national income from diabetes amount to hundreds of billions of international dollars in China and India. Clearly, novel therapies are required.
The burden for the patients, their care providers, and national economies drives the search for novel treatments, as new technologies may provide crucial human and economic benefits. One recent approach is continuous health monitoring, such as continuous glucose monitoring (CGM). A striking example is the recent proposal by Google: a biosensor implanted into contact lenses to determine tear glucose levels and transmit the data to a pump in the patient’s body. The pump will inject the required level of insulin, the missing hormone. This device circumvents the multiple daily blood tests and injections currently necessary with certain types of diabetes. Will the diabetes problem be solved simply by a contact lens? Well, life may be more complex.
Let’s have a look at what diabetes is to grasp the problem. There are two major forms of the disease: type 1, caused by the destruction of insulin-producing beta-cells in the pancreatic islets, and type 2, caused by insufficient insulin release and reduced efficacy of the hormone. Type 2, the major form, is often found in older patients, and a panel of lifestyle interventions and drug therapies are available as treatment. In contrast, type 1 surfaces earlier in life and requires hormone replacement therapy by insulin injections. As insulin is extremely potent and can cause brain damage and death by hypoglycemia, glucose levels have to be controlled very precisely, requiring constant blood sampling, which is a cumbersome burden for patients. Although fewer patients are afflicted with type 1 diabetes, still some 80,000 children develop the disease each year and numbers are on the rise. This situation provides a strong impetus to develop CGM via sensors that determine the amount of insulin to be injected. A number of different physicochemical methods have been explored for noninvasive approaches: surface-enhanced Raman, thermal or impedance spectroscopy, fluorescence, reverse iontophoresis, and photoacoustic or electromagnetic sensing. In general, these approaches are elegant but suffer from lack of specificity, problems in miniaturization, or provoke major local reactions impeding their use.
The accepted medical technology for CGM uses the protein glucose oxidase that generates an oxidation current upon reaction with glucose. Grafting the enzyme on an electrode allows for the determination of oxidation current and, hence, glucose levels. The first clinical devices were employed in hospitals in the 1970s, leading to the concept of the artificial pancreas—an implantable device measuring interstitial blood glucose that drives an insulin pump delivering the hormone via a subcutaneous needle. The first portable continuous glucose monitors to display real-time values became available in 2006. Recent data suggest that these devices not only lift an awesome burden from the patients but also improve their long-term health, an important argument for health insurance reimbursement. Even so, they still show a number of drawbacks such as considerable lag-time and less accuracy in hypoglycemia, a situation that may be life-threatening. Moreover, they work fine between meals but are not able to cope automatically with the rapid increase in glucose after a meal. As they tend to respond late and overdose, this may lead to a downward spike in blood glucose later on. A number of smart algorithms have been developed to improve CGM and insulin therapy. However, the current devices essentially work as an open loop because of the difficulty of finding an adequate pattern to master the complexity of life.
Can the biosensor, the centerpiece of the artificial pancreas, be improved? The current approaches rely on a small building block of cells, a protein. Can we respond better to complexity if we step up one level in the bio part and move to the cells? Indeed, the relevant cells or the micro-organ in our body has been shaped to its current function over a half-billion years of evolution. Thus, part of the work has already been done by nature, and we can perform the next step, the bioengineering, in somewhat less time. With this idea in mind, we recently started out to develop a novel type of hybrid bioelectronic sensor in a multidisciplinary approach between dialectologists, biologists, and microelectronics [1].
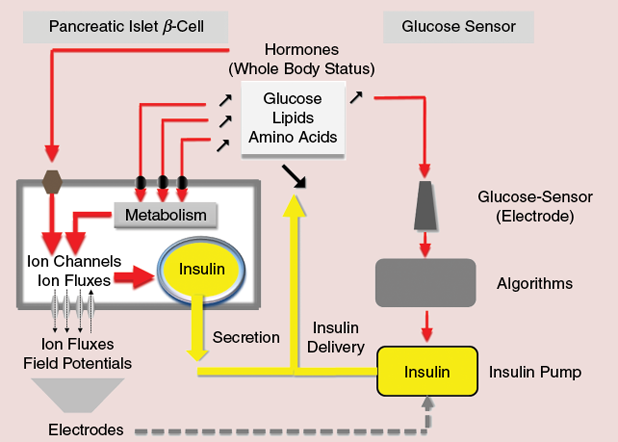
How do pancreatic islet cells work (see Figure 1)? The insulin-producing beta cells in islets completely metabolize glucose. In a way, they count each carbon of the molecule and produce a novel molecule, adenosine triphosphate (ATP), which is in precise correlation to the glucose levels outside the cell. ATP, in turn, will regulate a particular ion channel that depolarizes the cell, the KATP, and allows calcium influx through calcium channels. The calcium influx will trigger the release of the precise amount of insulin, adapted to glucose levels. However, food and nutrient homeostasis is much more than sugar. The beta cells will respond to and metabolize glucose as well as lipids and some amino acids and count their carbons. Therefore, unlike current sensors, our ß-cells release insulin whether we pinch a bit of our kid’s cotton candy or have a bite of foie gras.
More importantly, the cell also integrates a number of other signals, such as different hormones, that reflect the state and activity of the body. Jogging or trying to catch the train? You need your glucose and should not secrete much insulin. To this end, the adrenal glands release epinephrine, inhibiting ion channels and thus reducing ion fluxes. Therefore, the electrical behavior of the cells—their field potentials—is the first signal that integrates clues from different nutrients and takes the state of the whole body into account. Interestingly, the islets have two different major cell populations: beta-cells that secrete insulin and bring glucose down, and α-cells that secrete glucagon, a hormone that liberates glucose from the liver. α-cells behave exactly inversely of β-cells in electrical terms, and recording both will considerably increase precision. If we manage to keep these cells alive for days to weeks and capture these very tiny electrical potentials, we may obtain a highly suitable signal to determine the demand for insulin.
We were able to record these signals in islets obtained from mice, and most recently human donors, and found two distinct types of signals: short-lived action potentials, just as in nerve cells, lasting some tens of milliseconds, and slower undulations that we termed slow waves. Whereas the action potentials represent the activity of a single cell, the slow waves represent the further effect of the cell–cell interactions as found in the islet micro-organ. The signals are finely tuned by glucose within the physiological range and fully regulated by hormones [2]. Thus, cells have long followed the trend that generally comes up in the development of sensors: the implementation of multiparametric sensors to adapt to lifestyle and a multitude of daily events and to improve accuracy and safety, which is a major concern for use in humans.
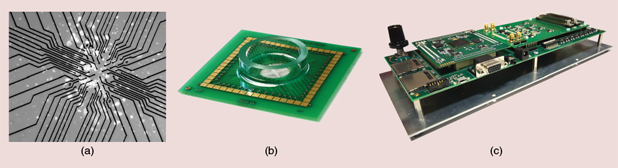
Recording quality is a challenge: it must be high enough to discriminate slight differences resulting from slow changes in the physiology. We used multielectrode arrays (MEAs) that contain multiple plates/electrodes to connect the cells to electrical circuitry [Figure 2(a) and (b)] [3]. MEAs typically have an arrangement of 60 electrodes with a diameter of between 10–20 µm. Cell preparations are cultured directly on the MEA. We designed custom integrated circuits able to process online and in parallel the 60 signals, in analog and then in digital mode. The circuits were embedded in an electronic board [Figure 2(c)] providing onboard data storage on SD cards and interfacing with standard peripherals using Universal Asynchronous Receiver Transmitter and Video Graphics Array protocols. Data processing is of two types: 1) the conditioning of raw recorded data to extract significant signal markers (action potential and slow waves) and 2) the evaluation of these markers to indicate changes in islets physiology. Thanks to the use of configurable circuits, evaluation can be adapted to the context and provides the user with quantitative information or go/no-go flags. The electronics were designed in low-power submicron technology, sized to ensure continuous and real-time processing on up to 60 channels. A computer interface is available to configure the system, launch recordings, and store raw or processed data in user-specific formats (Figure 3).

Currently, we are developing the technology for two distinct lab-on-chip applications: Diaßchip and Diaßsensor. Diaßchip will perform multiparametric testing of islets, to be used outside the human body for a number of defined applications. For example, one treatment of unstable forms of diabetes is the transplantation of islets. At this time, there is no quality control of donor islets that have to be transplanted within 6 hours. We have started a project with clinicians to compare transplantation outcome and islet quality. Another application concerns the differentiation of pluripotent stem cells intoß-cells. If this differentiation becomes possible and the whole process can be mastered reliably, diabetic patients, either of type 1 or of type 2, can be definitively cured by transplantation of these cells. Current procedures require the treatment of stem cells with very complex mixtures of biological factors, and their composition often changes daily. Diaßchip will be able to monitor their development on a noninvasive natural base and may also provide a useful element in quality control.
Our long-term goal, Diaßsensor, is even more ambitious and of high risk: the development of a novel and physiological sensor for an artificial pancreas and to free type 1 diabetic patients from cumbersome programming and complications inherent to glucose sensing only. Whereas islet transplantation needs several million donor islets, which are difficult to obtain, the sensor requires only a few dozen. Diaßsensor is essentially the core of a closed-loop controlling insulin delivery depending on the patient’s state and nutrient levels. Closed-loop control is very common in machinery, including cruise control in cars and temperature control at home [Figure 4(a)]. An artificial pancreas based solely on CGM is a step toward closed-loop control for diabetes but still misses essential points: glucose-only dependence and open-loop via the CGM [Figure 4(b)]. We expect Diaßsensor to act as a real closed loop with few, or ideally no, user inputs. It will therefore necessitate an extremely robust feedback control, able to handle both fast and slow changes in the patient’s physiology.
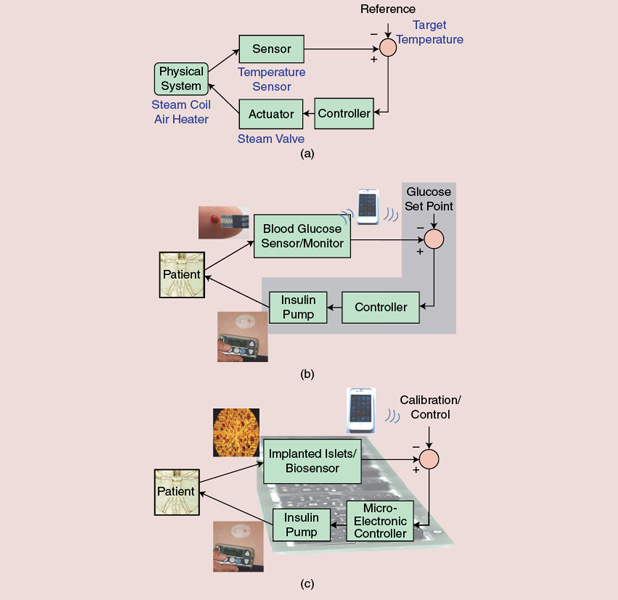
The whole-loop signal processing (conditioning, evaluation, and feedback control) has to be finely designed and flexible enough to suit most patients [Figure 4(c)]. Diaßsensor will require highly performing microelectronic technology, as the implanted device should be of minimum size and weight and ultralow power. Diaßsensor implies the use of cells that are foreign to the patient’s body with the risk of immune reactions. Recently, devices have been engineered that retrieve plasma from blood vessels and are positioned outside the patient’s body. Considerable progress has also been achieved in material sciences to provide adequate support that ensures the long survival of the cells. This field is a very active area, and considerable further progress can be expected in the near future.
It took almost 50 years to develop glucose sensors adapted to therapy, and they have revolutionized diabetes care. It is now time to go beyond the use of few constituents of cells, such as proteins, already marvels of evolution, and to exploit nature‘s more complex developments as given by a micro-organ such as the islets. Expertise gained in this field may also help neuroscience come up with operational biosensors and artificial organ parts to provide relief for a number of inborn or traumatic pathologies. In any case, the diabetes field, with its longstanding and well organized scientific, medical, and patient communities, should be able to benefit from interdisciplinary work in biomedical engineering.
References
- Y. Bornat, M. Raoux, Y. Boutaib, F. Morin, G. Charpentier, J. Lang, and S. Renaud, “Detection of electrical activity of pancreatic beta-cells using micro-electrode arrays,” in Proc. 5th IEEE Int. Symp. Electronic Design, Test and Application, DELTA ‘10, 2010, pp. 233–236.
- Q. V. Nguyen, A. Caro, M. Raoux, A. Quotb, J.-B. Floderer, Y. Bornat, S. Renaud, and J. Lang, “A novel bioelectronic glucose sensor to process distinct electrical activities of pancreatic beta-cells,” in Proc. 2013 35th Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC), 2013, pp. 172–175.
- M. Raoux, Y. Bornat, A. Quotb, B. Catargi, S. Renaud, and J. Lang, “Non-invasive long-term and real-time analysis of endocrine cells on micro-electrode arrays,” J. Physiol., vol. 590, no. 5, pp. 1085–1091, Mar. 2012.