Most people don’t worry about small cuts or wounds, because their bodies form clots to stop the bleeding. This process, called coagulation or hemostasis, requires certain blood cells, platelets, and protein clotting factors to interact correctly and form a clot to stanch the bleeding and begin repair of the damaged blood vessel.
However, people with bleeding disorders have blood that does not clot properly due to defects in their platelets or clotting factors: their symptoms may range from mild to life threatening. For patients with bleeding disorders, bleeding episodes can occur spontaneously, after trauma, or in association with menstruation. Fortunately, progress is being made in both diagnosing and treating such disorders. Researchers currently are concentrating on accurate and automated diagnostic methods as well as new high-tech treatments for bleeding episodes.
When Bleeding Is a Problem
The most common bleeding disorder is von Willebrand disease (VWD), a genetic disorder caused by a missing or defective von Willebrand factor, a protein that helps platelets stick to wounds. The next most common bleeding disorder is hemophilia, which is caused by a deficiency or dysfunction of blood clotting factor VIII or IX. Hemophilia is a recessive, X-linked condition, so it occurs predominantly in males.
When it is suspected that a person has a bleeding disorder, his or her physician may order several laboratory tests. The investigation of a bleeding disorder is usually a step-by-step process, with the results of some tests determining what further testing is needed. Diagnosing the specific bleeding disorder a patient has is typically based on family and patient history, in addition to clinical and laboratory testing. But these tests don’t always provide answers, and many patients end up with a diagnosis of “undefined bleeding disorder.”

“When we see people with bleeding disorders, they can have problems with platelets sticking, deficiencies in von Willebrand factor, problems with clotting proteins, defects in the ability to make fibrin, or an overactive clot-dissolving pathway,” says Catherine Hayward, a professor of pathology and molecular medicine at McMaster University in Hamilton, Ontario, Canada (Figure 1, right). Hayward says that, even today, people have bleeding disorders that we can’t diagnose. “Often, it’s a problem that sounds like it could be a platelet problem or VWD, but the tests for those come back normal.”
Because they are time consuming and require considerable expertise to interpret, some diagnostic tests are currently available only at specialized centers, and Hayward cautions that the more commonly available tests do not cover all the bleeding problems possible, “so not everyone who is investigated for bleeding gets a diagnosis or an answer for why they bled.”
Still, Hayward sees improvements to many of the diagnostic tests in use today. “For instance, the test for VWD used to involve adding an antibiotic that has nothing to do with bleeding because it made the von Willebrand factor stickier,” she says. “But we have new technologies to measure the function of von Willebrand factor by seeing how well it links onto the platelet protein—a key step in order to stop bleeding.” Hayward says this update in von Willebrand function testing is one of the biggest changes she’s seen over the last five years, but she would like to see more changes.
“I am hoping some of the more time-consuming and hands-on tests that we do will become automated,” she continues. “Right now, when our lab tests you for a platelet problem, they are working on your blood for an hour and a half to two hours, and not able to do anything else during that time. That’s a resource issue. But we can’t stop offering these tests, as they detect common and important causes of abnormal bleeding.”
Howard adds, “It’s important to have better tests for these diagnoses, because they do cause people to bleed excessively, either spontaneously or as a result of a trauma like childbirth or surgery. If one’s disorder is diagnosed and recognized, it can be life changing.”
When it comes to treatment, the degree and frequency needed depends on the condition and its severity. Typically, bleeding disorders are identified, monitored, and controlled to prevent excessive blood loss and reduce complications that may arise. For some bleeding disorders, patients receive infusions of clotting factor concentrates to prevent or treat bleeds. For other bleeding disorders, patients can self-administer topical products or nasal sprays to control bleeding, or they can receive fresh frozen plasma in a hospital setting.
Sussing Out “Wimpy” Platelets
In addition, there are exciting new diagnostic tools in the prototype stage, such as the “strength test for platelets” developed by biomedical engineers from the Georgia Institute of Technology and Emory University.

“Our approach is physics based, which is what makes it novel,” says Wilbur Lam, assistant professor in the Department of Pediatrics at the Emory University School of Medicine and the Wallace H. Coulter Department of Biomedical Engineering (Figure 2, right). Lam is a pediatric hematologist who also holds a Ph.D. in bioengineering. This training helped him realize the importance of the blood clot as a mechanical entity.
“You can think of a blood vessel as a pipe under a lot of pressure,” says Lam. “If there is a wound, suddenly the pipe has a hole and you’re leaking blood at high pressure. The body needs to patch up that hole, and that patch needs to be mechanically strong enough to withstand the high pressure that wants to break through it.”
Clot formation has a cellular component: blood cells stick to the wound as if plugging a hole in a dyke, and a clotting component creates a stable meshwork around those sticky cells. That meshwork is made of the protein fibrin. A series of enzymes and cofactors help build and stabilize a fibrin mesh around the platelets that have stuck to the wound. As blood clots form, platelets bind to fibrin and contract, shrinking the clot down to about 10% of its original volume. This clot contraction promotes wound closure and restoration of normal blood flow. The process can be deficient in several bleeding disorders but was previously difficult to measure.
Lam and his colleagues wanted to measure the pulling force of many platelets at one time, so they developed a gel spotted with tiny dots of fibrinogen, a sticky protein to which platelets easily bind. Platelets are able to demonstrate their strength on this testing ground when two of the protein dots are squeezed together. Lam and his colleagues inferred how strong someone’s platelets were by measuring the distance the protein dots moved. “You need to have platelets pull hard to have a successful blood clot,” says Lam. “Some platelets are wimpier than others, and patients with wimpier platelets are at a higher risk for bleeding.”
With the test, Lam and his colleagues were able to detect problems with platelet contraction in patients with known bleeding disorders, such as Wiskott–Aldrich syndrome, an immune deficiency disease that also leads to patients having a decreased ability to form blood clots. But Lam’s team also observed differences in some patients who had bleeding symptoms yet scored normally on standard diagnostic tests.
“Now that we have a system, we’re looking at making it even more robust and clinically adaptable, so one can use it in a hospital or clinical setting,” says Lam. “This could be another tool that hematologists use to diagnose people with bleeding disorders.”
Visualizing Blood Components
Researchers are also showing that technologies such as superresolution microscopy can be used to accurately and cost-effectively diagnose rare platelet diseases. Platelets help heal wounds through tiny granules that provide first aid to the vasculature. When there is damage, platelets are recruited to the site, and these granules release molecules for blood clotting. Platelet disorders include those that occur when these granules are malformed, are too few in number, or do not release the proper molecules.
Effective treatment depends on distinguishing exactly where the problem with the platelets lies. However, diagnosis has not been easy using traditional microscopy, as light microscopy struggles to resolve the individual granules and electron microscopy is time consuming and requires specialized equipment and expertise. Recently, however, researchers led by Daniel Cutler of University College London, have completed a proof-of-concept study based on structured illumination microscopy (SIM) to image platelet granules and diagnose rare platelet diseases.
Cutler and his colleagues used this superresolution microscopy coupled with image analysis software to automatically count the number of granules per platelet in blood samples and even collect data on each granule’s size and shape. The team successfully identified patients with Hermansky–Pudlak syndrome, a rare platelet disorder thought to affect one in 500,000 people. Their results showed that those with the disorder had about one-third as many granules as did controls.
SIM has many advantages over traditional microscopy for diagnostic purposes. Beyond the simple ability to resolve the granules, the use of automated granule identification ensures that analysis is unbiased, and the whole procedure is much less time consuming than electron microscopy.
“It took us about ten minutes to analyze ten times as many platelets as we could have done using electron microscopy,” says Cutler. “Automation not only releases all these highly trained individuals from what is a very routine task but also generates completely unbiased data that may be more reproducible from clinic to clinic.” But Cutler cautions that it might be some time before clinicians accept a brand-new diagnostic assay. “Rightly, they don’t want to jump into the unknown when important decisions about patient care are involved,” he explains.
A Better Way to Diagnose Joint Bleeds
Hemophilia is an inherited bleeding disorder that affects mostly males. Patients’ blood has difficulty clotting, and this leads to spontaneous bleeding into the joints and muscles. The result is often chronic pain and joint problems. Treatment typically consists of frequent intravenous clotting factor infusions, a procedure that can be very costly.
Annette Von Drygalski, director of the Hemophilia and Thrombosis Treatment Center at the University of California, San Diego, is working to develop more precise ways to diagnose bleeding in the joints. “Not all joint pains are due to bleeding, and clotting factor is very expensive,” says Von Drygalski. “I am interested in developing new point-of-care imaging modalities to detect joint bleeds before deciding on a course of treatment.”
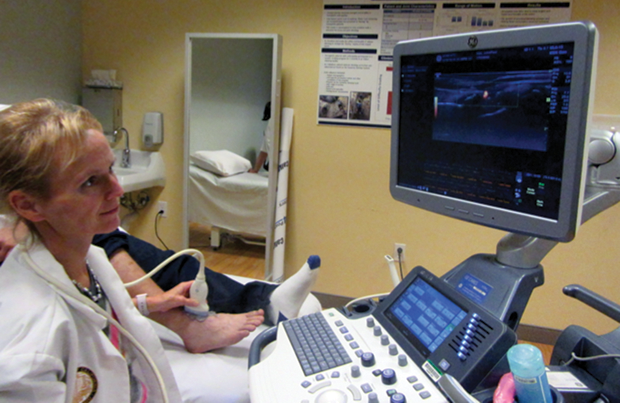
Von Drygalski and her colleagues are testing high-resolution musculoskeletal ultrasound to find blood accumulation in the joints. “There is no other good way, currently, other than ultrasound, to look at joint bleeds very quickly and right on the spot,” she says (Figure 3). Von Drygalski adds that the next step is getting this technology out of the clinic and into patients’ homes: “Maybe within the next three years we can develop the technology into a small, handheld device that patients can use in their own homes.”
The major advantage is that patients would not have to go to a clinic for all their pain; they could take an ultrasound image of a painful joint at home and send the image to their clinician. “Such a device would be the first point-of-care imaging in patients’ homes,” says Von Drygalski. “That would be really innovative.”
New Treatment Options on the Horizon
To stop excessive bleeding, clinicians sometimes place tiny metallic coils or liquid embolic agents into the affected blood vessel to block the flow of blood. However, both of these options come with potential problems and risks.
Metallic coils, for example, require blood clots to form around them in order to work. This can be problematic for people with clotting disorders or for those on anticoagulation medications. With liquid embolics, some time is required for the agent to emerge from a solution when injected into the blood vessel. In larger blood vessels, it might just get washed away before it has time to precipitate.

That’s where fitted hydrogel casts come in. Ali Khademhosseini, a professor at the Harvard Medical School and Brigham and Women’s Hospital and a faculty member of the Wyss Institute and the Harvard–Massachusetts Institute of Technology Division of Health Sciences and Technology (Figure 4, right), helped devise an easily injectable hydrogel that could stop uncontrollable bleeding. A recent paper provided proof-of-concept and preclinical evidence from animal models that the shear-controlled hydrogel can be delivered by catheters and injected into the blood vessels to form safe and sturdy blockages.
“We had previously developed this shear-thinning biomaterial to put externally on wounds to stop bleeding,” says Khademhosseini. “Because of the properties of the material, we thought it would be good for these internal bleeding cases, as well.”
The new material is a malleable hydrogel (Figure 5) that can mimic the function of platelet cells to promote blood clotting. When it is put under mechanical pressure—for instance, by pressing it through a syringe—it flows or “thins,” and when the pressure abates, it again solidifies to create a tight barrier. Importantly, it still works when normal blood clotting is impaired, such as in patients on blood-thinning medicine or those with clotting disorders.
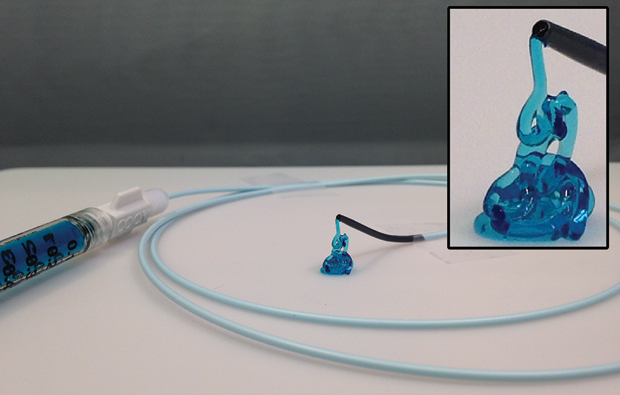
“I think with respect to the properties of the material and its delivery through a catheter, this is unique for bleeding disorders,” says Khademhosseini. Now, Khademhosseini and his team are trying to push this technology forward through the translational pathway. “This means doing the kinds of studies the [U.S. Food and Drug Administration] requires, like thorough toxicology analysis of the material and subsequent clinical trials,” he continues. “We are currently trying to either start a new company or license the technology to a larger, existing company.”
All over the world, researchers and clinicians are working on improving diagnostic tests and treatment options for people with bleeding disorders, hoping to understand what types of bleeding risks different types of patients face. The researchers behind these technologies estimate that, within the next three to ten years, patients could benefit from these advances in diagnostics and treatment.