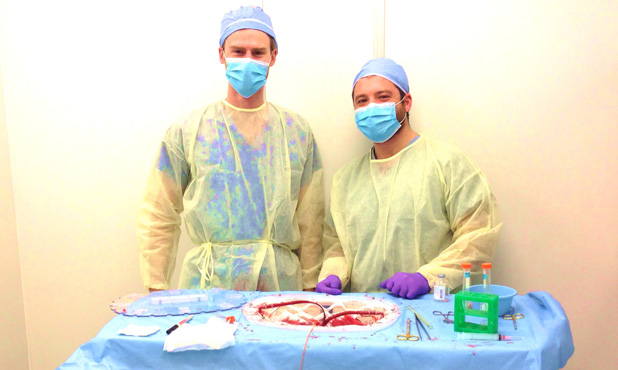
Above: “Preclinical testing of machine perfusion.” Photo courtesy of Korkut Uygun.
Replacing our failed body parts with new organs to achieve eternal life has captivated our imagination for generations. Seemingly futuristic, yet the pioneering steps were taken over a century ago, which led to the birth and advancement of organ donation and transplantation as we know it today. Organ transplantation has become such an essential and successful element of health care over the last two decades that it is easy to forget we are indeed far along on the path to having organs available on demand. As we have essentially solved the problem of how to replace organs, finding enough replacement organs becomes the major challenge.
The loss or compromise of organ function is one of the most hindering and costly problems faced by health care today. Total cost estimates range in hundreds of billions of dollars just for the United States alone [1], and the market for organ failure treatments is estimated at about US$80 billion/yr. At the same time, replacement of a failing organ is perhaps the most operational form of life extension therapy. In 2013, 28,952 organs were replaced in the United States, leading to an immediate improvement of the quality and length of many of these patients’ lives [2]. It is currently estimated that as many as 1 million deaths, or nearly 40% of all deaths, in the United States per year could be prevented by organ replacements.
The Organ Shortage Crisis
There are over 122,000 patients in the United States waiting for a transplantable organ, increasing by about 5% annually [3]. Several complementary tactics are currently pursued to overcome this organ deficit. These include public education efforts to increase donor registry enrollment and novel surgical techniques that aim to make more use of a single organ. Livers in particular, benefiting from an enormous regenerative capacity, can be split into two grafts to serve two smaller recipients. Donation from living donors is common fare for kidney transplantation, but even livers and lungs are being divided to support both the donor and the diseased recipient. Still, the benefits of these attempts are dwarfed by the ever-increasing number of patients waiting on the list.
Surprisingly, the true bottleneck causing the limited supply of donor organs is not the number of people who donate their organs, but rather that our current technology allows transplanting only a fraction of these organs—those that are in near-perfect condition. Organs deteriorate rapidly after death as a result of circulatory failure. For this reason, organ donation has traditionally been limited to brain death, which is formally defined as full, irreversible loss of neurological function including brain stem functions that control the most basic neurological activities. Sustained heart function in these donors after brain death (DBD) maintains a circulation of oxygen-rich blood through the organs and prevents them from dying along with the brain. Organs from brain dead donors can be procured with no warm ischemia—a state where the organ tries to perform at full functional capacity despite restricted blood and oxygen supply, effectively suffocating in the process. DBD transplants have led to excellent survival rates in recipients that are often in end-stage organ failure.
With an ever-increasing number of patients hoping to get a transplant, surgeons have been pushing the boundaries of acceptance criteria to tap into a large pool of potential donors outside of the brain death category. The most important group are donors from cardiac failure (DCD), ideally in a controlled hospital setting such that the warm ischemic time is short and well controlled. Over the last 10 years, more and more organs of these DCD donors are being used in an attempt to push back wait lists. However, even a short period of warm ischemia can be very detrimental to the organ and lead to chronic dysfunction, poor survival, and complications. Current cutoffs for warm ischemia range to up to 60 minutes depending on the organ, and simply doubling this limit could render tens of thousands of grafts transplantable and address at least today’s demand.
The Paradigm Shifts in Organ Preservation Technology
From a practical transplant perspective, current organ preservation was really invented in the 1970s with the introduction of specialized cold preservation solutions [4]. These solutions allowed placing the organ in a bag of ice-cold media, thereby reducing the metabolic activity and slowing the ischemic injury dramatically (Figure 1). This allowed the organ to be stored for hours rather than minutes, and enabled organ sharing and allocation even if only regionally. Still, in essence what we have achieved is a slowly, rather than quickly, suffocating organ. While for organs that are in perfect condition this does not present an issue, for marginally injured organs such as DCD grafts, this additional damage is too much. Current research therefore aims to shift the paradigm once more, and develop preservation methods that not only slow the deterioration, but actually allow us to resuscitate an organ that has been injured.
The ability to treat injured organs is just the beginning; expectations from novel preservation modalities include the ability to assess graft viability. A large number of marginal organs—we estimate a few thousand per year in the U.S. for all organs—are being discarded because there is no accurate way of assessing the function of the organ. As a result, borderline organs, which may have been transplantable, are being discarded for fear of failing and actually harming the patient. As of now, the cooling of organs slows down organ-specific functions and prevents us from testing organ viability prior to transplantation. A functional preservation method would facilitate this kind of testing and provide a diagnostic platform. Lungs, for example, have a very straightforward role of gas exchange over the alveoli, which is easily measurable in functional lungs outside of the body by simple gas analysis and are comparable to clinical levels.
Engineering an Artificial Body
The most notable attempts at keeping organs alive outside of the body came from the Nobel Prize-winning work of Alexis Carrel, who practiced the science he termed “organ culture.” Although Carrel preceded transplantation, his work was guided by the same principles governing transplantation research today and continues to inspire the organ preservation field. To maintain function of tissue there must be a continuous renewal of oxygenated medium containing nutritional substrates as well as a removal of catabolic products. The organ culture system, or machine perfusion as it has come to be known today, recapitulates many of the body’s functions with artificial components. A pump acts as the heart, pumping an artificial blood through a pseudo-lung (oxygenator) and circulating through the organ.
When Thomas Starzl performed the first liver transplants in the early 1960s, attempts were made to preserve perfusion of the liver during procurement, but at the time this was technically very difficult. When it was discovered that cold preservation worked fairly well, allowing surgeons to transplant organs many hours after procurement, and opening logistics for regional organ sharing, this became the standard operation procedure, which helped the organ transplantation field to grow rapidly. The rekindling of interest in extracorporeal machine perfusion is simply a result of reaching the limits of cold preservation technology and the need to use DCD organs.
Current machine perfusion attempts can be categorized into three broad embodiments, clustered according to the temperature of the system. Hypothermic perfusion (at ice cold temperatures, around 2–4°C) is an extension of the current methods: the organ is kept cold with a significantly reduced metabolism. Perfusion complements the preservation with a continuous renewal of the preservation solution, clearing metabolic waste products, and in some implementations oxygenating the media, although in the state of reduced metabolism this is not absolutely required. The advantage is the minimal risk in case of system failures—known to have happened in at least one clinical trial with warm perfusion—as the system automatically fails back to standard static cold storage. However, safety comes at the cost of physiological relevancy since at these temperatures and media, which do not support metabolic function, the organ is given little opportunity to function and recover itself from ischemic injury.
Simplicity and safety led to cold perfusion being the first clinical machine perfusion application and it is now in widespread use for kidney preservation. A large international, randomized, controlled trial definitively showed superiority of cold kidney perfusion in terms of function and post-transplant survival of the kidney. The complexity of other organs have led to a slower introduction, but the first pilot trial applying cold perfusion in DBD livers, performed at Columbia University, was promising, and a recent pilot trial from Zurich, Switzerland, showed good results after transplantation of livers from DCD donors using cold perfusion. These results suggested that perfusion in the cold can improve preservation of marginal donors to achieve results similar to DBD livers [5].
Functional preservation uses antithetical approaches to maintain warm temperatures and allow the organ to function normally such that it can recover from injury, in particular in terms of its energy reserves. This recovery allows the organ to retain function and repair additional injury in the aftermath of transplantation. Normothermic, or body temperature, perfusion is the idealistic version that aims to fully mimic the body’s physiology. The organ is generally kept at 37°C and perfused with either full blood or a nutrient-rich media featuring an oxygen carrier, often simply red blood cells, since artificial alternatives are rare and controversial. While this approach has immense potential, sustaining full physiological function ex vivo is not an easy feat, considering the complex organ physiology and the substantial variation between organs from donors of different backgrounds and states of injury. The body has countless feedback mechanisms between all tissues regulating every aspect of organ metabolism at multiple levels, which are not only difficult to replicate but is also to a large extent not yet fully discovered. Moreover, the need for whole blood or equivalent vastly increases system complexity, cost, and possible points of failure. Nevertheless, normothermic organ perfusion for several hours has provided good results. In Toronto, Canada, lungs of marginal quality have been preserved for several hours at body temperature in perfusion systems and transplanted with excellent results and similar trials are ongoing for the liver and heart.
A practical optimum may lie in an intermediary perfusion temperature, known as subnormothermic machine perfusion. This is an approach that we refined in our lab over the last 7 years, where elements from an originally normothermic system were iteratively eliminated to reduce redundant complexities until we converged to an effectively unregulated room temperature of 21°C. Since metabolic demand is reduced at subnormothermic temperature, we found that we do not need an oxygen carrier in these systems and simply dissolving the gas into the perfusion solution is sufficient to sustain function, thereby eliminating the need for oxygen carriers and simplifying the pseudo-lung design. Yet this temperature is sufficient for the mitochondria to function. The nutrient-rich media supports a markedly higher metabolism than cold systems and the liver has the opportunity to recover. In animal models, subnormothermic perfusion has shown great superiority and enabled transplantation of 60-min warm-ischemic livers with perfect survival compared to 0% with traditional cold storage. To place this in context, this improvement would make about 6,000 extra livers transplantable and eliminate waiting for liver transplant in the United States and Europe. In a recent study, we demonstrated feasibility of this system in human livers that were discarded for transplantation and clinical trial preparation has begun [6].
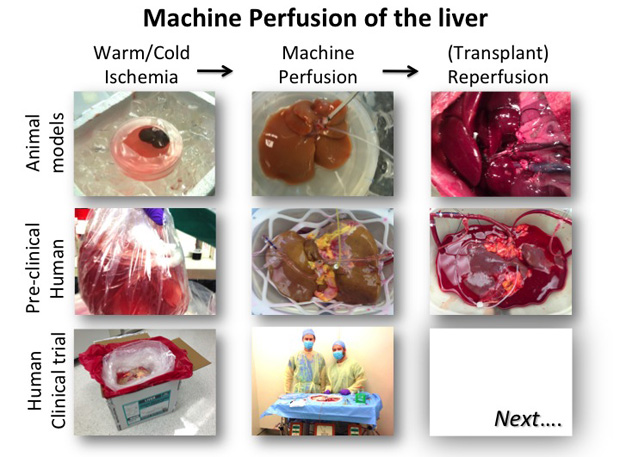
Potential of Organomatics
For an engineer, observing the field of functional organ preservation develop is a bit like watching a documentary on steam engines or automobiles. Development of a new field and solving first-order problems such as temperature are inspiring, yet the absence of sophisticated, integrated engineering solutions creates a great sense of impatience, especially with thousands of patients perishing on the organ waiting list.
Creating an artificial body to provide for an organ ex vivo is extreme engineering. Countless factors influence the dynamics of organ function. Various feedback mechanisms that take place in vivo need to be mimicked ex vivo by tandem employment of sensors, data analytics, and automation beyond what perfusion systems in use today can provide. Yet an overview of perfusion systems of commercial availability or in development will show even basics such as feedback pH control are rare features. Watching perfusionists manually add bicarbonate to media is inevitably reminiscent of a driver in the 19th century starting a car with a crank handle.
We term the art and science of analyzing an organ’s function across the scales of omics Organometrics, and the endeavor to optimally manipulate function to enhance its health and transplant success, Organomatics. These are more aspirational goals than current reality; however a successful Organomatics platform would be an enormously powerful tool in organ preservation, allowing not just long term ex vivo maintenance of the organ but also accurate assessment of organ function at various levels and manipulation of function to fit therapeutic requirements. Equipping this artificial body with sensor networks would allow for real-time imaging of micro perfusion quality and assessment of functional parameters at chemical, cellular, and tissue scales. Soft sensors could be used to complement these, predict missing data, and interpolate data that is not available in real time, or estimate the viability and probability of transplant success. Armed with such information, we could then stop assuming all organs are identical and use constant set points, and instead utilize model predictive control schemes that maximize tissue viability and apply treatments that are finely tuned to the individual organ, its physiology, and, if injured, etiology —practicing personalized medicine at the organ level.
Realizing Organomatics in the clinic will require a close collaboration between clinicians and engineers. It would be an overstatement to claim it’s even in its infancy. But as early organ perfusion systems make a rapid entry into clinical practice, a vast amount of clinical data will become available that will fuel this area and lead to tremendous insight into isolated organ function and regulation, which can be directly fed back into clinical practice. It’s the right time, too. As the eye of “big data” turns to health care, data of every kind will be brought to light and we will need a horde of people with skills and knowledge both in medicine and data sciences, a rarity today.
The future of functional organ preservation with machine perfusion appears laden with opportunity. We will learn to build artificial bodies that can sustain organs alive, diagnose and treat the injured organs automatically, and bring back the dead ones as spare parts for the tens of thousands of patients on waiting lists today and many more in the future. Surgeons can’t realize this dream alone, neither can scientists or engineers. People who can do it all, wanted.
Acknowledgments
Funding from the US National Institutes of Health (R01EB008678, R01DK096075, R01DK084053), and the Shriners Hospitals for Children is gratefully acknowledged.
Disclosures
The authors of this manuscript have conflicts of interest to disclose: Authors are inventors on patent applications WO/2011/ 002926 (KU); WO/2011/35223(KU), and MGH Disclosure 22743 (BGB, KU). KU has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Dr. Uygun’s interests are managed by the MGH and Partners HealthCare in accordance with their conflict of interest policies.
References
- R. Langer and J. P. Vacanti, “Tissue engineering,” Science vol. 260, pp. 920-926, 1993.
- Organ Procurement and Transplantation Network.
- Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2012 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, 2014.
- F. O. Belzer and J. H. Southard, “Principles of solid-organ preservation by cold storage,” Transplantation, vol. 45, pp. 673-676, 1988.
- P. Dutkowski, A. Schlegel, M. de Oliveira, B. Müllhaupt, and P. A. Clavien, “HOPE for Human Liver Grafts obtained from Donors after Cardiac Death,” J. Hepatol., vol. 60, pp. 765-772, 2013.
- B. G. Bruinsma, H. Yeh, S. Ozer, P. N. Martins, et al., “Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation,” Am J Transplant, vol. 14, pp. 1400-1409, 2014.