The nervous system of the human brain consists of diverse types of neurons that are interconnected. The enormous number and heterogeneity of neurons and their connections has made understanding the brain a difficult task, and it has long been a dream in basic and clinical neuroscience to be able to not only read the brain’s neuronal activity but also to have control over distinct sets of neurons.
In recent years, optogenetics has been booming in the neuroscience community. It offers a new set of molecular tools for controlling the behavior of specific types of neurons (cells) by using light. It has successfully overcome the drawbacks of traditional brain stimulation approaches, including electrical, physical, and pharmacological stimulation methods. This unique technology is able to achieve cell-type-specific, precise, transient, chemical-free delivery and reversible manipulation of neuronal activities. The development of optogenetic technology has revolutionized how neuroscientists dissect the neuronal circuits to better understand brain function.
The first major optogenetic protein used to modulate neuronal activities is Channelrhodopsin-2 (ChR2), a light-gated ion channel. When blue light at 480 nm is illuminated on ChR2, it will open the cation channels and induce cellular depolarization. Neurons engineered to express ChR2 fire an action potential in response to a pulse of blue light on the millisecond timescale most relevant for neural computation. No exogenous chemical cofactors or reagents are required for the whole process.
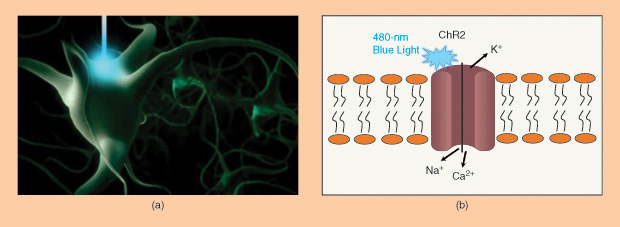
The Development of Optogenetics
Early in 1979, English biophysicist and neuroscientist Francis Crick suggested that the major challenge facing neuroscience was “a method by which all neurons of just one type could be inactivated, leaving the others more or less unaltered.” Crick speculated that light might serve as a control tool, but, at the time, neuroscientists didn’t have a clear approach to making specific cells respond to light. Over the past few decades, a number of studies have been conducted to develop methods for optically stimulating specific cell types. For example, optical uncaging of neurotransmitters with ultraviolet laser pulses was already a powerful method for activating neurons, due to its high speed and spatial precision, but it had not yet been adapted to a neuron-type-specific form. Then came the discovery that the expression of a single protein—ChR2—can mediate light-sensitive cation currents, enabling robust and temporally precise control of neural activity both in vitro and in vivo. The light-sensitive probes are mostly natural microbial light-driven ion pumps, known as opsins, causing a membrane depolarization, membrane hyperpolarization, or a change in intracellular signaling.
Opsins actually have been studied since the 1970s. Bacteriorhodopsin, for example, discovered in the early 1970s in the archaeon Halobacterium salinarum, pumps protons out of cells in response to green light. In the late 1970s, halorhodopsin, an orange light-driven inward chloride pump, was discovered in the same organism. In the early 1980s, the first microbial sensory rhodopsin was discovered: a phototaxis receptor also in the same organism. Since then, both rhodopsin ion transporters and sensory rhodopsins have been found to be widespread in a diversity of archaea, bacteria, fungi, and algae, where they serve photosynthetic and signaling purposes. In 1999, Hildebrandt and colleagues showed that one halorhodopsin from Natronomonas pharaonis (NpHR) functioned best, at chloride levels comparable to those in the mammalian brain. In 2003, Georg Nagel, Ernst Bamberg, and colleagues announced the discovery of ChR2 [1], the opsin that drives phototaxis in the green alga Chlamydomonas reinhardtii. They showed that ChR2 was a light-gated cation channel that, when illuminated, allows positively charged ions to pass into cells in which it is heterologously expressed.
By August 2005, Ed Boyden and Feng Zhang from the Karl Deisseroth Lab at Stanford University reported stable and safe expression of ChR2 in mammalian brains by using the lentiviral gene [2]. This was the first time opsins were brought to neuroscience research. Their article demonstrated that upon introduction of the ChR2 gene without any other components, neurons became precisely responsive to light in a millisecond timescale. The term optogenetics was coined around that time. It was first used in Karl Deisseroth’s article in 2006 [3] to refer to the use of light to image genetically expressed reporters of neural function as well as to perturb genetically targeted neurons.
Significant efforts have been made in subsequent years to lift the optogenetic approach to a level of broader applicability. By 2007, the technology had been adapted to achieve precise control of the behavior of mice. The Guoping Feng Lab, then at Duke University [and at the Massachusetts Institute of Technology (MIT)], began to make the first transgenic mice expressing ChR2 in neurons around that time as well. In the same year, Boyden’s lab and Deisseroth’s lab reported in parallel that the halorhodopsin could support the light-driven silencing of neurons. By 2010, channelrhodopsin, bacteriorhodopsin, and halorhodopsin all had proven capable of turning neurons on or off, rapidly and safely, in response to diverse colors of light.
The bioengineering of existing opsin genes from different microorganisms generated a variety of chimeric rhodopsin versions with modified properties regarding tracking, kinetics, and light responsivity. In parallel, opsins from various species were screened for their optogenetic suitability. Channelrhodopsin from different species extended the optical spectrum of cellular excitation, and the light-driven proton pumps enabled neuronal silencing in addition to NpHR. Besides its role in neuroscience, other research fields recently have started to illuminate ChR2-targeted tissues such as cardiomyocytes and embryonic stem cells, proving the universal capabilities of optogenetics. Continuous progress in optical technologies and opsin engineering will not only further consolidate optogenetics as an excellent experimental tool but will also lay the foundation for potential future therapeutic applications.
How Can You Design Your Own Optogenetic Experiment?
In general, the experimental design strategy under consideration will need to take into account the region and cell type of interest as well as the scientific question under consideration. One should keep in mind that the use of optogenetic devices requires the delivery of light to the tissue region of interest. The implantation of optical hardware is generally required for light delivery.
The first question a researcher interested in using an optogenetics approach for his or her experiments must answer is: Which cellular action do I want to control? The principal options currently available are:
- membrane depolarization—to activate neurons, such as ChR2
- membrane hyperpolarization—to silence neurons, such as NpHR
- modulation of intracellular biochemical signaling pathways—to mimic the action of hormones or neuromodulators on cells, such as the optoXRs, where X specifies the particular pathways being optically modulated, and R represents receptors.
Other related parameters one should consider include the light wavelength, kinetics of the opsin activation, etc. Optogenetic molecular tools generally utilize endogenous retinoids to sense light only in a specific spectrum, ranging from far red to far blue. Similarly, optogenetic devices have been engineered with a range of kinetics of on and off activation, with the generally more sensitive opsins requiring longer kinetic timescales.
The second question a researcher might ask is: What type of cells do I want to control, and how I can express the opsin specifically in that cell type? Gene coding for light-sensitive proteins can be delivered to target cells by transfection, viral transduction, or the creation of transgenic animal lines. Expression can be restricted to cells of interest using specific promoters or recombinase-based conditional systems. The use of viral vectors for gene delivery can allow targeting of specific cells without specific promoters, for example, to target neuronal populations based on their topological connections. Lenti and adeno-associated viral vectors have been used successfully to introduce opsins into the mouse, rat, and primate brain. Additionally, these have been well tolerated and highly expressed over long periods of time, with no reported adverse effects. However, a major downside of viral expression systems is the maximum genetic payload length; only promoter fragments that are small (less than ~4 kb), specific, and strong may be used, and these are rare. This limitation may be skirted using Cre-driver animals and Cre-dependent viruses. The use of transgenic or knock-in animals obviates viral payload limitations and allows for tighter control of transgene expression using larger promoter fragments or indeed the endogenous genome in full via knock-in. The first transgenic opsin-expressing mouse line was generated using the Thy1 promoter and has been widely used. Several groups have subsequently also generated transgenic mouse lines directly expressing opsin genes. The University of North Carolina at Chapel Hill Vector Core Facility is now providing vector services for researchers to get optogenetic constructs.
The third major question in designing an optogenetic experiment is: Where is my region of interest, and how should I deliver light there? Currently, many different light-delivery protocols have been developed and applied to various studies for precise manipulation of cellular activity. The researcher can adapt those approaches to his or her own design, which mainly depends on the region of interest for the study. In vitro, stimulation has been conducted with filtered light from mercury arc lamps, lasers, and light-emitting diodes (LEDs). In vivo, stimulation of behaving/anesthetized animals have been conducted mostly with laser light delivered to the opsin-transduced tissue via optical fibers mounted on top of or inserted through chronically implanted cannulae. The illumination can be temporally controlled using an ultrafast shutter with a constant light source. Furthermore, infrared-triggered miniaturized LEDs have been used recently, which could open a new direction in optogenetic research. In addition, light-patterning approaches can be used to selectively illuminate a subset of cells. One approach uses digital micromirror-based devices to reflect dynamic user-controlled light patterns onto specific cells or regions of cells. On the other hand, two-photon scanning microscopy can be used for targeted activation of single ChR2-expressing cells with high temporal control.
Finally, researchers should consider how to read out the neuronal manipulation results. Combining appropriate readout strategies with the optogenetic inputs is essential for facilitating fundamental studies on neural circuits. The major readout approach to measure the neuronal activity induced by optogenetic manipulation is electrophysiological recording. Such a system combines a single-unit recording electrode(s) with optical fibers carrying light illumination and is collectively called an optrode. Recently, more sophisticated approaches have emerged; these employ either silicone multisite electrodes or movable electrode arrays combined with optical fibers for more flexible interrogation of neural activity in vivo. However, many neuroimaging methods have been adapted to detect the effects of optogenetic manipulations. Two-photon imaging, voltage sensitive dye imaging, laser speckle contrast imaging, and other optical imaging modalities have been demonstrated successfully. Furthermore, blood-oxygenation-level-dependent functional magnetic resonance imaging readouts have been integrated with optogenetic manipulations for studying neuronal connectivity at a whole-brain level, in a manner previously not feasible with microelectrodes and most optical imaging methods [4]. Meanwhile, traditional behavioral testing has been widely used to directly assess the effect of modulating cellular activity in animals. The researchers shall decide which measurements to read out the optogenetic manipulation results and design the integrated system accordingly.
Applications of Optogenetics
Optogenetics has been widely applied to probe neuronal circuits in diverse animal models, including Caenorhabditis elegans, flies, zebrafish, mice, rats, and primates. Studies have been using the technology to map neuronal projections, examine neuronal plasticity in the interested pathways, and simulate disease-related circuits. Although currently it is not feasible for direct clinical application in patients, several groups have made significant progress on understanding human brain disease mechanisms in animal models by integrating optogenetic toolkits to the study.
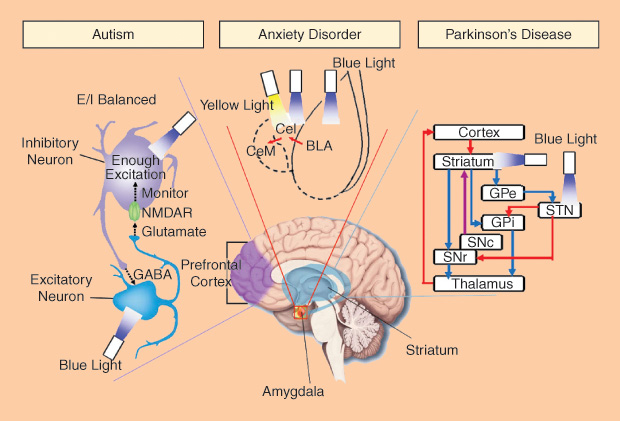
Autism
Autism is a developmental brain disorder with a prevalence of about one to two cases per 1,000 people worldwide. Although autism has a strong genetic basis, environmental factors may also cause autism. It has been linked to altered neural network organization and synapse connection, but the complete mechanism has not been well understood. In the cerebral cortex, neural networks execute information processing with high accuracy and speed, using a mechanism called balance of excitation and inhibition (E/I balance). The behavior deficits in autism are hypothesized to arise from elevations in the ratio of cortical cellular excitation to inhibition within neural microcircuitry. Traditional electrophysiology and optical imaging methods cannot perform empirical tests of this hypothesis.
Using optogenetic tools, researchers specifically controlled the activity of excitatory neurons or inhibitory neuron within the mouse medial prefrontal cortex and investigated the relation of E/I balance to the social deficits [5]. They found that an elevation of cellular E/I balance elicits, a profound impairment in cellular information processing and further specific behavior impairments. A compensatory increase of inhibitory neurons excitability partially rescued the social deficits. As a potential diagnostic method, electrophysiology recording demonstrated an increased power density of high-frequency range (30–80 Hz) during the elevation of cellular E/I balance, which has also been observed in clinical conditions in autistic patients. The optogenetic toolbox creates a new way for the investigation of severe psychiatric disease symptomatology on the cellular, circuit, and behavioral levels.
Anxiety Disorder
Everyone has had an anxious experience under stress. While most people can return to normal, some do not. Excessive feelings of anxiety and fear are typical characteristics of an anxiety disorder. Anxiety disorders are partly genetic but may also be due to the use of alcohol and caffeine. In our brain, the amygdala is the key structure in emotional control. It has neural connections with the hypothalamus, hippocampus, and cingulate gyrus. In the amygdala, glutamatergic basolateral amygdala (BLA) can excite GABAergic neurons in centrolateral (CeL) amygdala, which provides feed-forward inhibition onto CeM GABAergic neurons (output of amygdala). The BLA-CeL-CeM circuitry is considered to be important in emotional processing, but its detailed mechanisms in anxiety disorder are unclear.
Using optogenetics, the BLA-CeL-CeM circuitry has been investigated at the level of specific projection types and cell types [6]. Researchers found that temporally precise optogenetic inhibition of BLA terminals in CeL increased anxious behaviors. By way of contrast, optogenetic stimulation of the same projections presented an acute but reversible anxiolytic effect. Furthermore, direct optogenetic stimulation of BLA somata showed a distinct anxiogenic effect. These results demonstrate that specific neural projections, beyond neuron types, are critical in the circuit function relevant to neuropsychiatric disease.
Parkinson’s Disease
Parkinson’s disease (PD) is a chronic and progressive movement disorder. Nonmotor symptoms, including emotional and cognitive problems, have also been reported. The mechanism of PD is still unknown, and there is currently no cure, although there are treatment options. The death of dopaminergic neurons in the substantia nigra (SN, part of basal ganglia system in the midbrain) mainly contribute to the motor symptoms of PD. It is well known that two parallel basal ganglia pathways (direct-pathway for activation and indirect-pathway for inhibition) dominate the motor planning and action selection. However, the mechanism of parkinsonian state formation in the basal ganglia circuitry remains unknown.
With the help of optogenetics, researchers can specifically control the stimulation of direct- and indirect-pathway medium spiny neurons [7]. For normal mice, bilaterally exciting the indirect-pathway elicited a parkinsonian state meanwhile activation of direct-pathway reduced freezing and enhanced locomotion. For model mice of PD, stimulating direct-pathway completely rescued deficits in freezing, bradykinesia, and locomotor initiation. These findings also represented a potential therapeutic strategy, i.e., activating the direct pathway, for motor deficits in PD.
Because the neuron loss in SN alters the activity of the subthalamic nucleus (STN), high-frequency (>90 Hz) stimulation of STN is performed as deep brain stimulation (DBS) treatment to reduce motor deficits. Generally, DBS may simultaneously increase or decrease the activity of different target neurons around the targeted brain volume. This makes the therapeutic mechanism of DBS in PD very difficult to understand. When researchers applied the optogenetic toolbox to systematically activate or inhibit distinct circuit elements in STN of parkinsonian rodents [8], they found the therapeutic effects of DBS within STN can be accounted for by directly stimulating the afferent axons projecting to this region. Optogenetics provides a precise technological toolbox for systematic deconstruction of individual components in disease circuits.
References
- G. Nagel, T. Szellas, W. Huhn, S. Kateriya, N. Adeishvili, P. Berthold, D. Ollig, P. Hegemann, and E. Bamberg, “Channelrhodopsin-2, A directly light-gated cation-selective membrane channel,” Proc. Natl. Acad. Sci. U.S.A., vol. 100, no. 24, pp. 13940–13945, 2003.
- E. S. Boyden, F. Zhang, E. Bamberg, G. Nagel, and K. Deisseroth, “Millisecond-timescale, genetically targeted optical control of neural activity,” Nat. Neurosci., vol. 8, no. 9, pp. 1263–1268, 2005.
- K. Deisseroth, G. Feng, A. K. Majewska, G. Miesenbock, A. Ting, and M. J. Schnitzer, “Next-generation optical technologies for illuminating genetically targeted brain circuits,” J. Neurosci., vol. 26, no. 41, pp. 10380–10386, 2006.
- N. Li, J. E. Downey, A. Bar-Shir, A. A. Gilad, P. Walczak, H. Kim, S. E. Joel, J. J. Pekar, N. V. Thakor, and G. Pelled, “Optogenetic-guided cortical plasticity after nerve injury,” Proc. Natl. Acad. Sci. U.S.A., vol. 108, no. 21, pp. 8838–8843, 2011.
- O. Yizhar, L. E. Fenno, M. Prigge, F. Schneider, T. J. Davidson, D. J. O’Shea, V. S. Sohal, I. Goshen, J. Finkelstein, J. T. Paz, K. Stehfest, R. Fudim, C. Ramakrishnan, J. R. Huguenard, P. Hegemann, and K. Deisseroth, “Neocortical excitation/inhibition balance in information processing and social dysfunction,” Nature, vol. 477, no. 7363, pp. 171–178, 2011.
- K. M. Tye, R. Prakash, S. Y. Kim, L. E. Fenno, L. Grosenick, H. Zarabi, K. R. Thompson, V. Gradinaru, C. Ramakrishnan, and K. Deisseroth, “Amygdala circuitry mediating reversible and bidirectional control of anxiety,” Nature, vol. 471, no. 7338, pp. 358–362, 2011.
- A. V. Kravitz, B. S. Freeze, P. R. L. Parker, K. Kay, M. T. Thwin, K. Deisseroth, and A. C. Kreitzer, “Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry,” Nature, vol. 466, no. 7306, pp. 622–626, 2010.
- V. Gradinaru, M. Mogri, K. R. Thompson, J. M. Henderson, and K. Deisseroth, “Optical deconstruction of parkinsonian neural circuitry,” Science, vol. 324, no. 5925, pp. 354–359, 2009.



