Article Submission
Submit articles for publication in JTEHM through IEEE Author Portal. All submissions must follow the IEEE JTEHM templates. Upon acceptance, authors are encouraged to include either a video abstract or a high-resolution image for both Xplore and JTEHM.
A length of 8 pages of text, including references, is recommended for papers. Communication manuscripts are limited to 5 pages (4 for text and 1 for acknowledgment and references). Supplemental material does not count toward the page length.
Article Processing Fees
As a Gold Open Access journal, JTEHM charges article processing fees.
Article Processing Charge (APC): US$1995 – Effective 1 January 2024
For papers submitted in 2023, the Article Processing Charge (APC) is US$1950 plus applicable local taxes.
For papers submitted in 2024, the APC is US$1995 plus applicable local taxes.
Discounts
The following discounts apply:
- IEEE Members receive a 5% discount.
- IEEE EMB Society Members receive a 20% discount.
Discounts do not apply to undergraduate and graduate students. These discounts cannot be combined.
Corresponding authors from low-income countries (as classified by the World Bank) may be eligible for discounts or fee waivers. Eligible authors of accepted papers should contact the editor-in-chief.
Questions?
More information can be found in our FAQs.
Author Instructions for Manuscript Preparation
August 2020
JTEHM publishes translational research papers and findings in all stages of technology translation to healthcare and clinical applications, emphasizing expected or demonstrated impact in patient care and medical practice. JTEHM welcomes submission of all types of translational research falling upon the spectrum explained below.
The articles in this journal are peer reviewed in accordance with the requirements set forth in the IEEE Publication Services and Products Board Operations Manual. Each published article is reviewed by a minimum of two independent reviewers using a single-anonymous peer review, where the identities of the reviewers are not known to the authors, but the reviewers know the identities of the authors. Submitted articles are screened for plagiarism before review.
Word Choice
We prefer that authors use inclusive language. For example, please use non gendered terms when possible, and replace the terms “master” and “slave” with “parent” and “child” or “primary” and “replica.”
Manuscript Types
Papers
These manuscripts have a length of up to 8 pages of text, including references (8 pages is recommended). Papers must include the sections below.
Download the Paper Template
Download the LaTex Version of the Paper TemplateStructured Abstract: Limited to 250 words with the following headings (headings do not contribute to the word count).
- Objective
- Methods
- Results
- Discussion/Conclusion
Index Terms: Up to 5 keywords in alphabetical order.
Clinical and Translational Impact Statement: An impact statement of no more than 30 words. The statement should address how the reported findings serve to improve human health (or provide a path to improving health), and articulate a strategy for how the reported device, diagnostic, therapeutic, or service may be integrated into a clinical or home healthcare setting. The authors are encouraged to review the NIH Clinical Spectrum (see below) and indicate the best category for the reported work: Early/Pre-Clinical Research, Clinical Research, Clinical Implementation, or Public Health. Any submission without this statement will be returned to the author without review.
Body: The main manuscript body. This is organized at the author(s)’ discretion with the exception of the following main headings.
- Introduction
- Methods and Procedures
- Results
- Conclusion
- References
- Acknowledgments and Appendices/supplemental material (not required, but encouraged)
Communications
Short manuscripts of 5 pages maximum (4 for text and 1 for acknowledgment and references). Communications may be peer reviewed through the normal process; or they may follow an expedited review process at the Editor-in-Chief’s discretion. Ideal Communications are accompanied by additional material such as video, databases, and executable codes of any models as provided by the authors. Communications should report on new results on novel technologies in early translational development phase, early clinical implementation and validation, or discussion of emerging challenges and unmet needs.
Download the Communication Template
Structured Abstract: Limited to 150 words with the following headings (headings do not contribute to the word count):
- Objective
- Methods
- Results
- Discussion/Conclusion
Index Terms: Up to 5 keywords in alphabetical order.
Clinical and Translational Impact Statement: An impact statement of no more than 30 words. The statement should address how the reported findings serve to improve human health (or provide a path to improving health), and articulate a strategy for how the reported device, diagnostic, therapeutic, or service may be integrated into a clinical or home healthcare setting. The authors are encouraged to review the NIH Clinical Spectrum (see below) and indicate the best category for the reported work: Early/Pre-Clinical Research, Clinical Research, Clinical Implementation, or Public Health. Any submission without this statement will be returned to the author without review.
Body: The main manuscript body organized in the following manner:
- Introduction and Clinical Need
- Results
- Discussion
- Methods
- Future Directions and Potential Clinical Impact
- Acknowledgment (not required, but encouraged)
- Appendix/Supplemental Material (if desired)
Letter to the Editor
A Letter to the Editor is a brief report or commentary that is within the journal’s scope and of particular interest to the community, but not suitable as a standard research article. It can represent the opinion of an individual or a small group of individuals/stakeholders. Letters must be limited to 500 words and will be published on the JTEHM website without any open-access publication fee.
Letters to the Editor may be edited for clarity or length and may be subject to peer review at the Editors’ discretion. To contribute, please contact the Editor-in-Chief.
Download the Letter to the Editor Template
Contact the Editor-in-Chief
Appendices/Supplemental Material
JTEHM strongly encourages authors to submit appendix material such as videos, data files, models and programs, detailed figures, and any additional information. This information will be posted on the JTEHM website with links to the published paper in IEEE Xplore.
NIH’s Translational Science Spectrum
Translation is the process by which pure technologies and observations become workable solutions, bettering peoples’ lives and bolstering communities. In order to emphasize the nonlinear, multidirectional nature of the path to implementation, the NIH has replaced its Four Stages of Clinical Translation with the Translational Science Spectrum. The Spectrum is broken down into the five broad areas. It is these categories which must be referred to in your manuscript’s Clinical Impact Statement.
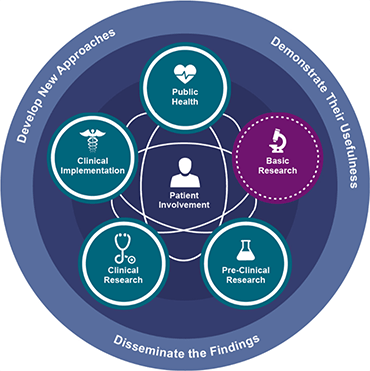
Basic Research
Basic research involves scientific exploration aimed at revealing fundamental mechanisms of biology, disease, or behavior, and technological modalities. All translational science is built upon and informs basic research.
Preclinical Research
Preclinical research links basic research with human health and medicine. During this stage, model interventions may be designed to further understand the issue and how it might be treated. Testing is carried out using cell or animal models, tissue samples, or computer-assisted simulations. The interventions tested may include drugs, devices, or diagnostic tools and their interactions within living systems.
Clinical Research
Clinical research includes studies to better understand a disease in humans and relate this knowledge to findings in cell or animal models; testing and refinement of new technologies in people; testing of interventions for safety and effectiveness in those with or without disease; behavioral and observational studies; and outcomes and health services research. The goal of many clinical trials is to obtain data to support regulatory approval for an intervention.
Clinical Implementation
Clinical Implementation involves the adoption of demonstrably useful interventions into routine care. In addition, this stage includes the research to evaluate the results of clinical trials and to identify gaps in care and areas in need of improvement within the given intervention.
Public Health
Public health research involves the study of health outcomes at the population level to determine the effects of diseases and health issues, and the failures, success, and long-term consequences of current health interventions. At this stage, population-level research helps scientists assess current interventions, and identify and develop new ones.
