Ventricular tachycardia and ventricular fibrillation are the most common causes of sudden cardiac death in patients with chronic heart failure (CHF). Furthermore, as the next generation of therapeutics for heart failure is developed, it is increasingly important to have reliable and clinically relevant pre-clinical models for characterizing the effects of drugs/treatments.
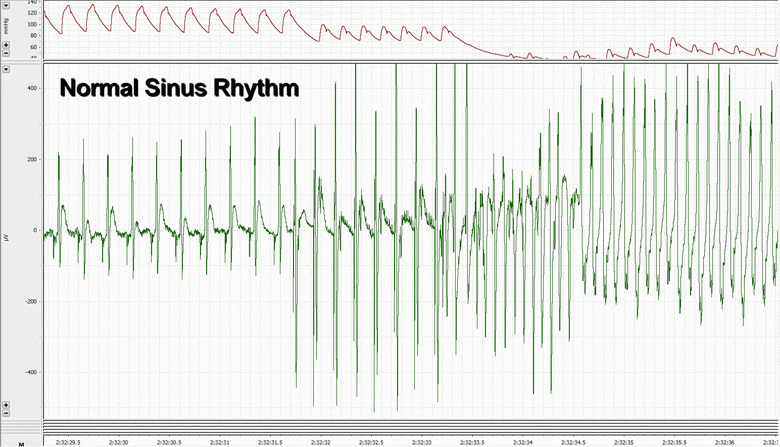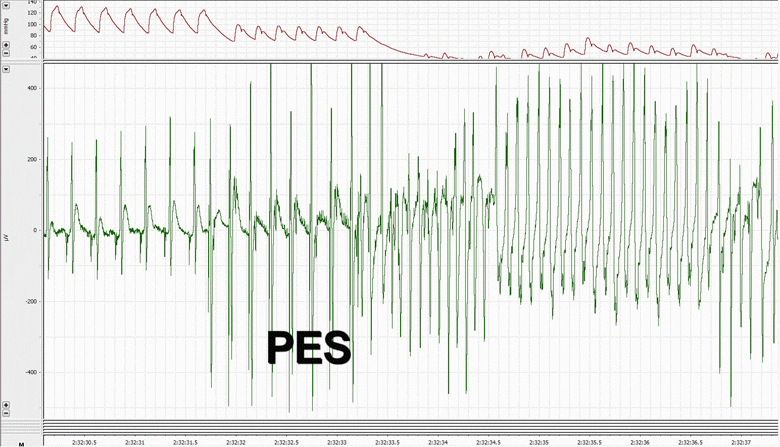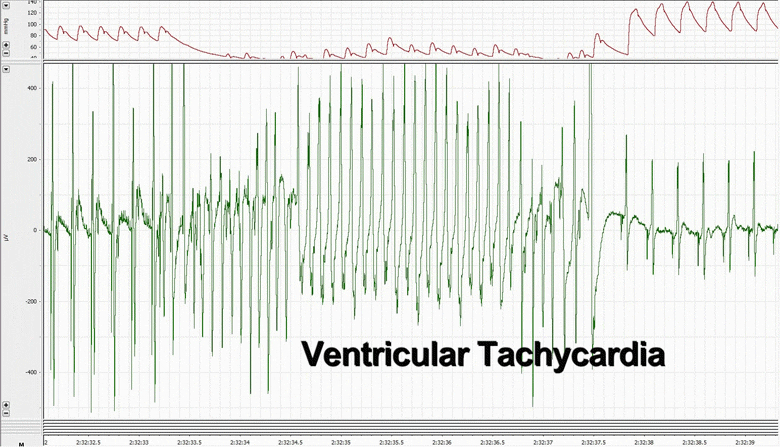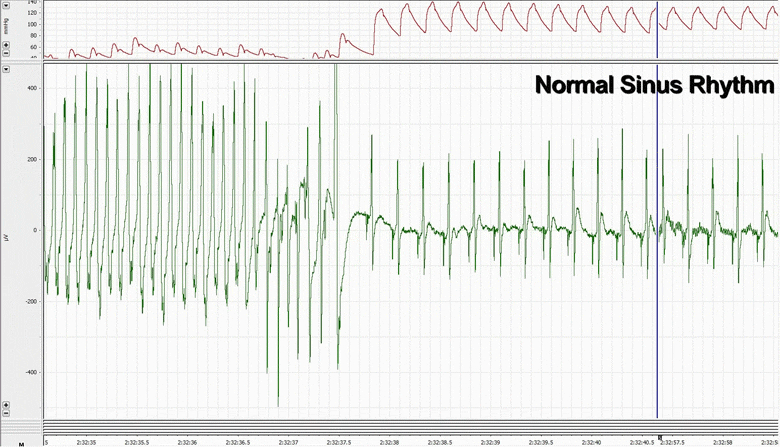
In this work, we investigated the electrophysiologic consequences of ischemia in our in vivo rat model of chronic heart failure. After echocardiographic and hemodynamic confirmation of heart failure from left coronary ligation, we performed recordings from the epicardium of both CHF rats and SHAM-operated controls. Monophasic action potentials, voltage amplitudes, and electrical activation sequences were collected, in addition to arrhythmia-induction studies via programmed electrical stimulation to induce ventricular tachycardia.
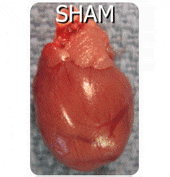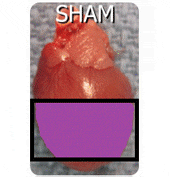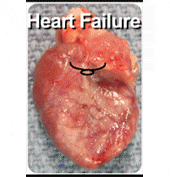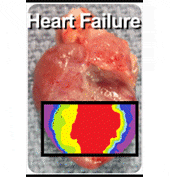
Rats with CHF were found to have the expected echocardiographic and hemodynamic changes including elevated left ventricular (LV) end-diastolic pressure, decreased LV ±dP/dt, ejection fraction, peak developed pressure, and prolonged time constant of LV relaxation Tau. In terms of electrophysiologic parameters, the CHF rats showed decreased voltage electrogram amplitudes, decreased monophasic action potential amplitudes, increased repolarization heterogeneity, and increased incidence of induced-sustained ventricular tachycardia. We were able to define three subtypes of tissue within an infarcted myocardium, namely healthy, border, and scar. Using concurrent monophasic action potential recordings and electrocardiogram recordings, we were able to investigate the initiation of ventricular tachycardia from the cellular level, and found delayed afterdepolarizations.
We propose that this in vivo rat model in combination with our electrophysiologic methods could be used to study the genesis of ventricular tachycardia in CHF and the mechanisms by which sudden cardiac death occur, as well as to determine the arrhythmogenic potential of new treatments, such as drugs, biologics, stem cells, and devices, in CHF.