Elizabeth A. Calle, Mahboobe Ghaedi, Sumati Sundaram, Amogh Sivarapatna, Michelle K. Tseng, and Laura E. Niklason, Yale University, Volume 61, Issue 5, Page: 1482-1496
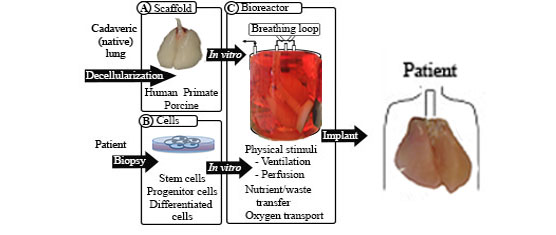
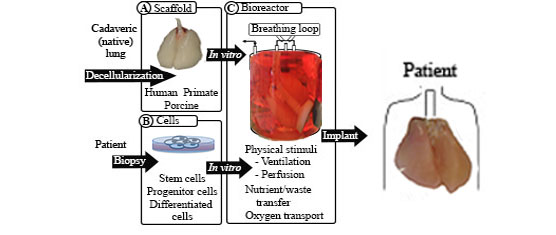
Whole organ engineering, including that of whole lungs, requires three central components: a scaffold, cells to populate the scaffold, and a bioreactor in which to cultivate the populated scaffold. The scaffold must provide the structure of the organ and access to all cell compartments. The cells used to populate the organ must include epithelial, endothelial, and interstitial or mesenchymal cells. These cells may be differentiated cells, they may be derived from pluripotent stem cells, or they may be organ-specific progenitor cells. The scaffold is initially mounted in the bioreactor for both cell delivery and subsequent delivery of nutrients, oxygen, and physical stimuli to the seeded construct. Key challenges in scaling whole lung engineering from rodent lungs up to human-sized organs include identifying a reliable and easily procured scaffold source that can be sterilized without deleterious consequences to the biological scaffold. The key epithelial and mesenchymal cell types to populate the scaffold must also be identified. The derivation of these cells from pluripotent cell types would allow the creation of tissue with minimal immunologic consequences and would allow for more extensive expansion of the cells either prior to seeding or within the scaffold. Whether a well-populated scaffold is fit for implantation into large animals or humans will ultimately be determined by the effectiveness of endothelial cell seeding of the vascular compartment. Without a sufficiently endothelialized vasculature, an engineered organ will not be viable in vivo. To aid development of the overall construct in vitro, bioreactor conditions that provide adequate nutrient and waste transfer, oxygen transport, and mechanical stimuli such as ventilation will also be necessary. Though the challenges are great, progress has been rapid so far. Continued effort in this area, particularly that which is interdisciplinary, will assist in overcoming the many challenges that remain in whole lung organ engineering.
http://medicine.yale.edu/anesthesiology/people/laura_niklason-3.profile