Andrew J Casper, Dalong Liu, John R. Ballard, and Emad S. Ebbini
Volume: 60, Issue: 10, Page(s): 2751-2759
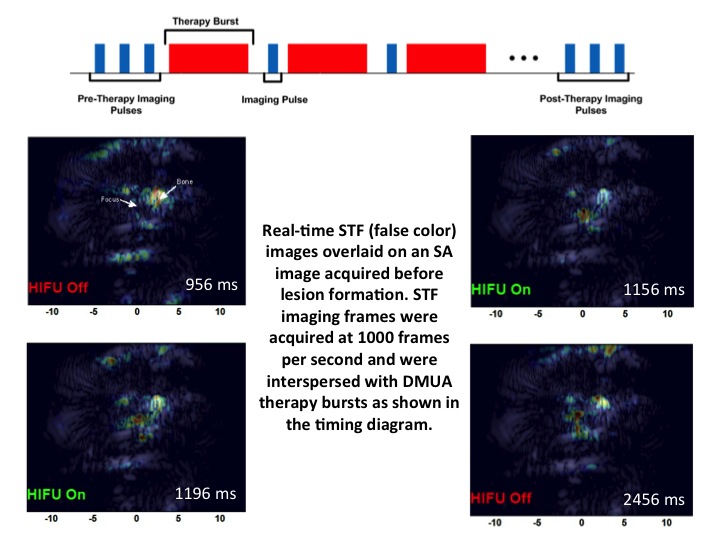
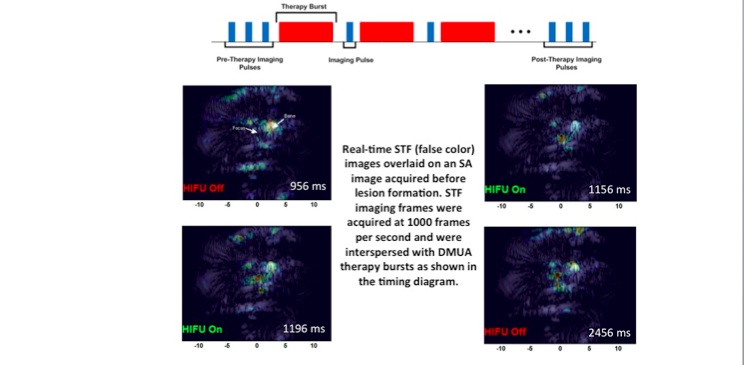
Focused ultrasound surgery (FUS) is gaining wider acceptance as a form of noninvasive “bloodless” surgery in hospitals and clinics worldwide. Image-guidance was the catalyst that led to this wider acceptance since the mid 1990s. In addition, advances in transducer array technologies have allowed for the use of phased-array techniques to generate complex therapeutic beams for improving the precision and efficacy of the surgery in the presence of tissue heterogeneity. Our group has introduced the concept of dual-mode ultrasound array (DMUA) systems as a new paradigm in FUS, which allows for using the same therapeutic array for imaging the tissue response to FUS beams. The uniqueness of this approach stems from the inherent registration between the imaging and therapeutic coordinate systems and the precise interrogation of tissue response from the location of the therapeutic focus. In this paper, we have presented the first in vivo demonstration of the advantages of DMUA imaging in real-time monitoring of lesion formation dynamics. A prototype DMUA was used to target the hind limb of a Copenhagen rat to form a small lesion in muscle tissue near the femoral bone. Two modes of DMUA imaging were used in this experiment: 1) Synthetic-aperture (SA) imaging for obtaining anatomically correct images of the target region, and 2) Single transmit focus (STF) imaging for monitoring lesion formation dynamics. SA DMUA imaging was compared with conventional imaging using a diagnostic linear array probe on a commercial scanner and example results were reported in the paper. High frame-rate STF DMUA imaging was used for spatial and temporal localization of cavitation activity within the target tissue in response to the application of pulsed FUS (pFUS). STF frames where taken before, during, and after the application of the pFUS focus in a synchronous manner with the therapy bursts (see illustration). M-mode images from STF frames (shown in the paper) clearly demonstrate the high degree of spatiotemporal localization of cavitation activity during lesion formation. STF imaging is also useful in identifying any strongly scattering objects in the path of the pFUS beam, e.g. scattering from the femoral bone in this case.