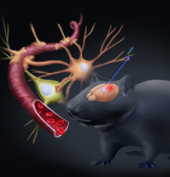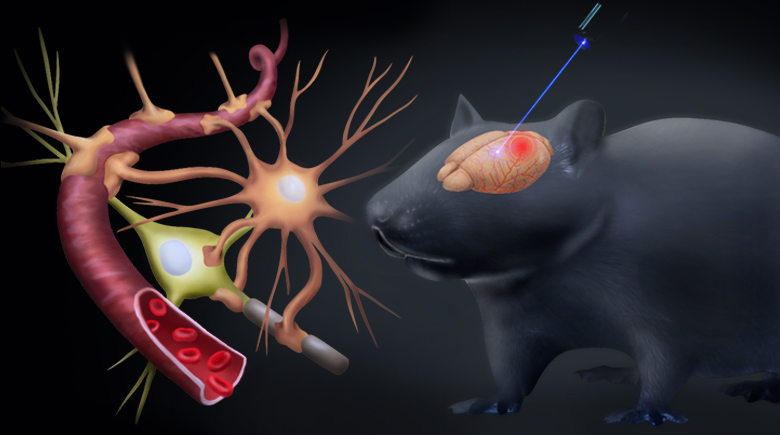
Brain stimulation has recently been reported to be a promising technique for stroke intervention through directly modulating the metabolism in brain. However, most brain stimulation techniques have limitations in precision and are not able to induce cell-specific modulation on the target. The cell-specific excitation or inhibition is required in order to understand the underlying mechanisms and justify its clinical applications in neurological diseases. Optogenetics provides a cell-specific approach to modulate the neuronal activity at the millisecond timescale. Though there have been several studies on the neuron-specific stimulation using optogenetics in chronic stroke, showing positive functional recovery, so far, there isn’t a report on the acute stage of stroke.
Sensorimotor neurons were transfected with channelrhodopsin-2 and excited by a 473-nm laser. The photothrombotic stroke was induced in the ipsilateral parietal cortex and the targeted area for modulation remained intact. Optogenetic stimulation was carried out within 2 hrs after stroke in the modulation group. Laser speckle contrast imaging was used to measure the cerebral blood flow and the neurovascular response to optogenetic stimulation with the first 24 hrs after stroke. Our results demonstrated that excitatory activation of neurons in ipsilesional sensorimotor cortex during acute stroke could successfully reduce the expansion of the ischemic core and protect the neurovascular function after stroke. The neuroprotective effects were consolidated by both histological and behavioral results. Improved collateral circulation could possibly contribute to these neuroprotective effects. The results implied the neuron-specific modulation as a potential therapeutic intervention for the acute ischemic brain injury.