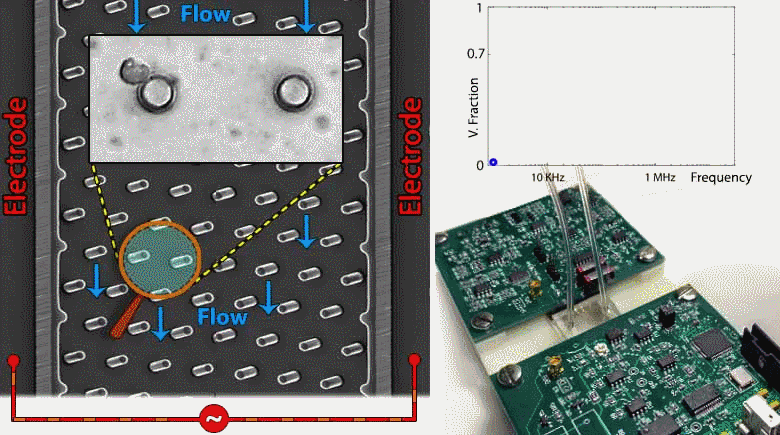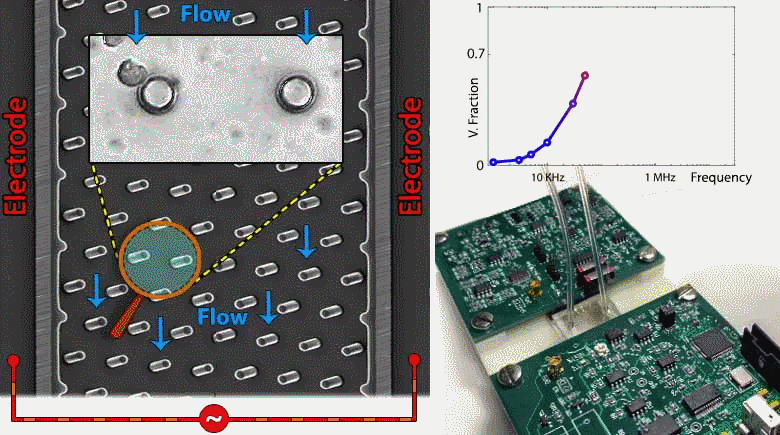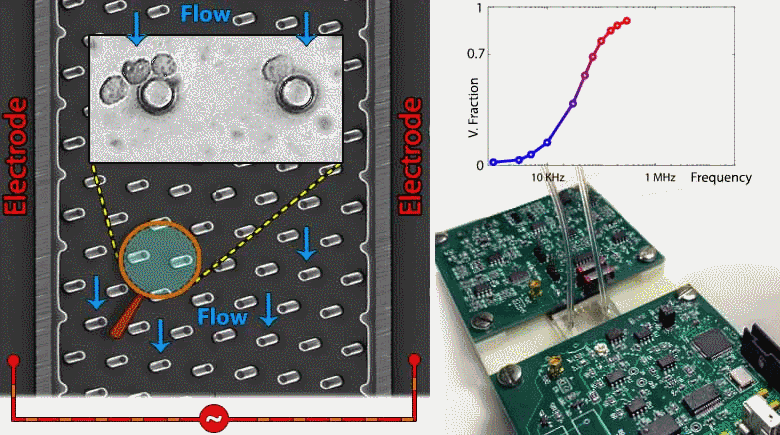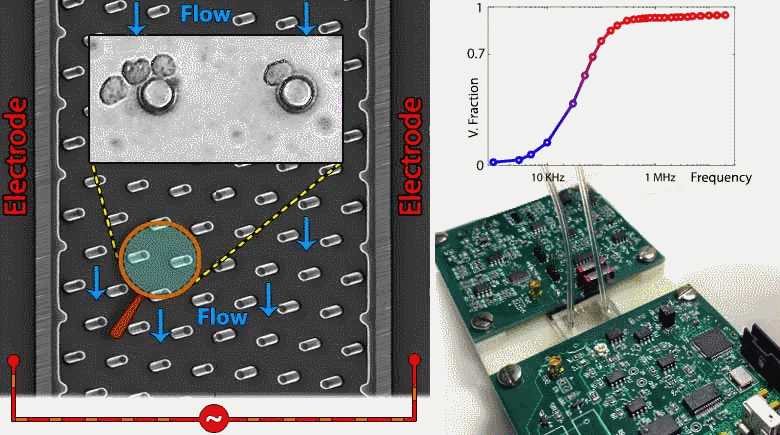
Microfluidic devices are emerging within several biomedical diagnostic and therapeutic applications for the purpose of accurately focusing and trapping cells or particles under electrical, magnetic, acoustic and inertial force fields. However, current methods to monitor effectiveness and location of cell or particle trapping under microfluidic force fields require cumbersome imaging to be conducted by a trained operator, with limited ability to rapidly trigger downstream separation decisions. To address this need, we present an embedded circuit for an impedance sensor that directly interfaces to the microfluidic chip for monitoring cell or particle trapping based on the level and frequency response of parasitic voltage drops measured at the AC power levels and frequency ranges used for during electrokinetic manipulation. Through automated fitting of the acquired impedance spectra from the microfluidic chip to an equivalent circuit model, the parasitic voltage drops at each device region can be quantified to assess the microfabricated device geometry, such as width, surface area and surface charge distribution, as well as the overall channel architecture. Based on this, the device geometry and stimulation conditions can be systematically altered to maximize fraction of the applied voltage available for electrokinetic cell manipulation and to temporally alter the downstream separation characteristics of trapped cells. While the current study is focused on impedance-based assessment of microfluidic devices designed for contactless dielectrophoresis, we envision that this principle can be applied towards other microfluidic structures for electrokinetic manipulation, as well as for manipulation under acoustic or inertial force fields. Such an embedded circuit approach utilizing on-chip monitoring to trigger downstream events enables active control of microfluidic processes for applications in single-cell focusing, cytometry, and separation.