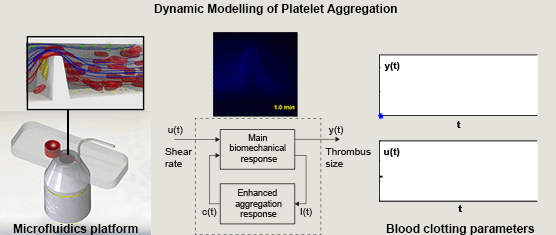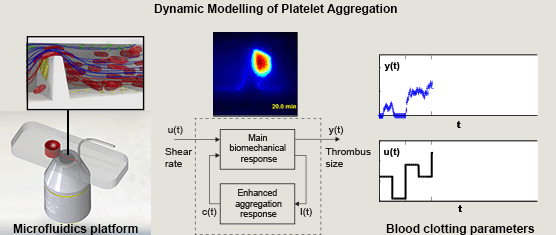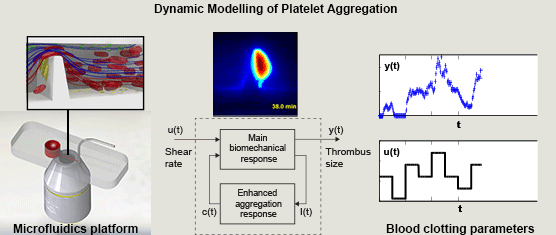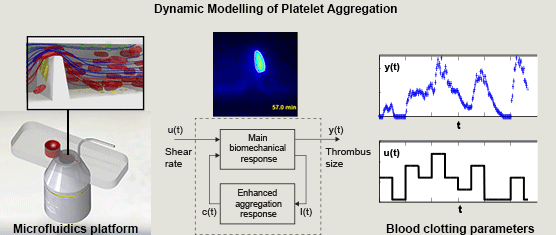
Miguel E. Combariza, Xinghuo Yu, Warwick S. Nesbitt, Arnan Mitchell, and Francisco J. Tovar-Lopez, RMIT University, Australia, Volume 62, Issue 7, Page: 1718-1727
The development of blood clots at sites of atherosclerotic plaque rupture -arterial thrombosis- remains a major cause of patient death worldwide despite decades of intensive research. The recent application of new microfluidic technologies and methods has facilitated significant progress in the understanding of the fundamental mechanisms governing blood platelet function and how these parameters affect pathological thrombus formation. In-line with these new bioengineering approaches, the application of nonlinear dynamic systems analysis holds particular potential to extend our understanding of the complex interplay between mechanical and biochemical factors that underlie this complex biological phenomenon.
In this paper we propose a simple nonlinear dynamic model of the main dynamics of platelet aggregation/disaggregation observed experimentally in a novel microfluidic device that approximates a severe arterial stenosis. We apply dynamic systems theory (system identification) to explore the dynamics of the biomechanical platelet aggregation response to a range of shear stress rates, inhibiting blood-born chemical pathways of platelet activation (ADP, TXA2 and thrombin).
We demonstrate that the proposed model is able to replicate experimental results with low variation, and suggest that the reduced set of model parameters has the potential for the application of high throughput analysis of platelet function. The approach detailed in this study is the first to demonstrate the application of control theory principles to dynamically control and limit platelet aggregation, and is the first to address the control of reversible aggregate formation. This systematic approach to extracting simple parameters from the rather complex blood flow experiments renders the platform a more attractive candidate for point of care diagnostics that can be operated and interpreted by non-specialists.