Over the past decades, the advantages of robot-assisted surgery over conventional laparoscopy have become increasingly apparent. As the performance demands on the robotic surgery tools grow, so does the need for a deeper understanding of the physical phenomena that govern both the tools and the tissue treated, as well as the control laws for the precise attainment of the surgical objectives.
A common surgical technique, electrosurgery, relies on the use of high-power radio-frequency currents to actively heat organic tissue, allowing it to be denatured, coagulated, desiccated, fulgurated, or incised. One of the key advantages of electrosurgery is its simultaneous cutting and coagulation capability, providing blood stoppage for complex surgical tasks. However, a key disadvantage of human-operated electrosurgery is the tendency to damage nearby tissue due to an excessive influx of heat. The effects of this damage are not apparent to the surgeon during operation and appear only hours to days after being inflicted.
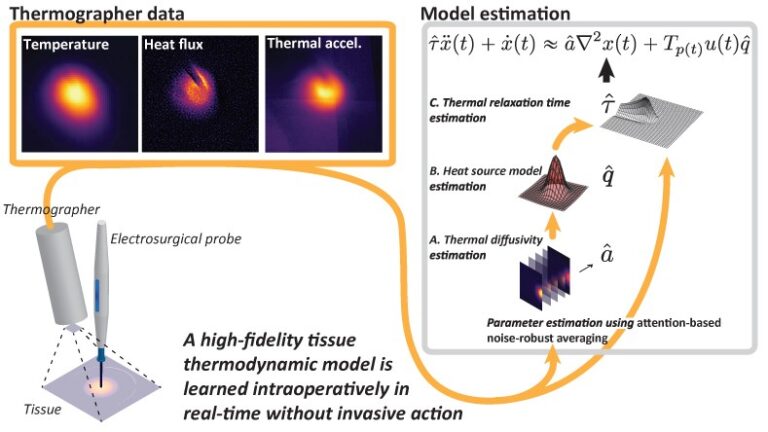
Motivated by this gap in understanding of patient-specific tissue thermodynamics needed to achieve minimally invasive surgery with improved post-operative recovery, we demonstrate the suitability of the hyperbolic Maxwell–Cattaneo model of heat propagation to support a high-fidelity thermodynamic representation of the live tissue response to electrosurgical impact.
In addition to validating this model on live tissue data, we present a novel thermodynamic parameter estimation framework for energy-based surgery on live tissue. This framework allows for modeling tissue thermodynamics in real time, allowing for prediction of thermal damage impact to the tissue and damage-conscious planning of electrosurgical procedures. Our approach relies solely on thermographer data and is therefore minimally invasive and applicable in situ. We verify the method’s accuracy through its application to simulated data based on porcine muscle tissue, as well as in vivo liver tissue with a comparison of the results with those from the literature.