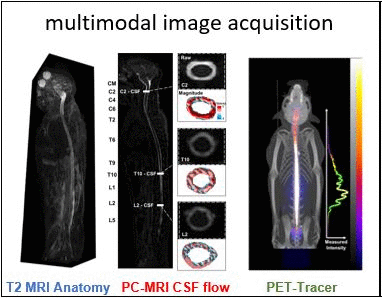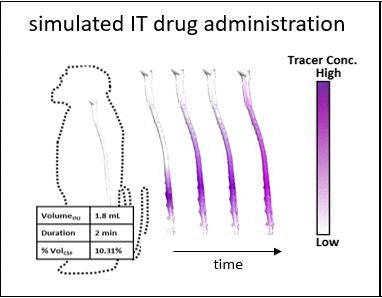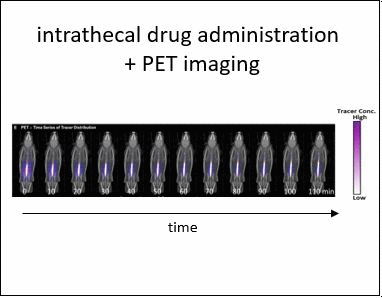
Intrathecal (IT) delivery is a drug administration method that bypasses the blood-brain barrier by injecting therapeutic molecules directly into the central nervous system. In vivo tracer infusion experiments performed in cynomolgus monkeys dispel the common belief that in intrathecal drug delivery, agent distribution is confined to a narrow region close to the injection site, thereby undermining the efficacy of the method. Our multimodal imaging confirms that IT administration with proper infusion settings can achieve wider dispersion than has been reported previously. At high infusion rates, the tracer reached the cervical region after only two hours, demonstrating rapid and wide biodistribution.
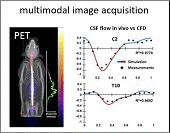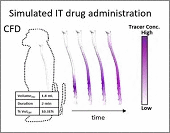
However, choosing optimal infusion settings is not intuitive, nor can they be determined by trial-and-error animal experimentation due to the large number of variables and high cost. The solution: computational models offer a rational framework to predict biodistribution of intrathecally infused agents. The same in vivo nonhuman primate imaging data also provided validation for a computational fluid dynamic model for the prediction of drug distribution following IT injection. Tracer dispersion was predicted with subject-specific simulations. The CFD predictions of drug dispersion showed close spatio-temporal agreement with tracer biodistribution acquired in vivo with PET imaging.
The experiments reinstate IT delivery as a viable administration method for targeting molecules to the spine or the brain. The proposed computational methodology enables rational design of novel therapies for neurological diseases that require reliable, efficient and safe delivery of therapeutic agents to specific target sites in the central nervous system. The study also suggests intrathecal administration as a promising modality for novel treatments such as enzyme replacement or gene therapies targeting neurodegenerative diseases.