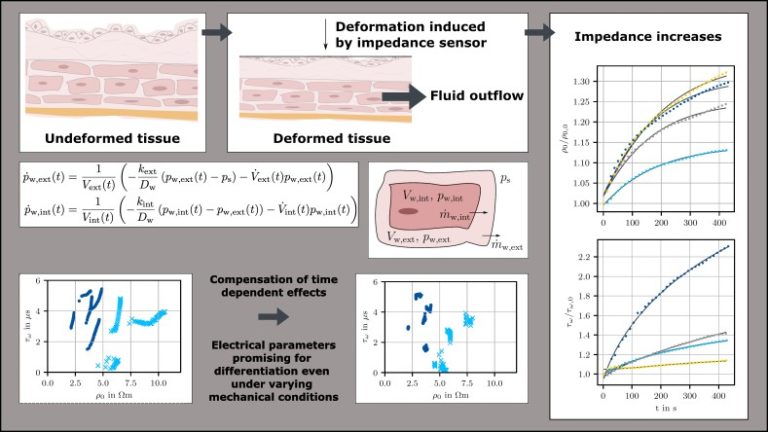
Bladder cancer recurrence remains an important issue after urological surgeries because the removal of tumors is based solely on the endoscopic video and the surgeon’s evaluation of the tissue. Sensor measurements, such as electrical impedance measurements, can detect altered physical properties of the tissue and aid the surgeon in determining which areas to preserve and which ones to remove. However, in a highly deformable organ like the bladder, mechanical deformations of the tissue can lead to extra- and intracellular fluid flows from stretched or compressed areas. As ion-containing tissue fluid is a major contributor to impedance, this phenomenon significantly changes the tissue’s electrical behavior, such that a compressed healthy bladder wall may exhibit the same electrical characteristics as an uncompressed tumor.
To address this issue, we developed a novel fluid model based on differential equations that takes into account extra- and intracellular flow under compression. The model is based on the fluid content within the individual tissue compartments and its outflow via diffusion. The outflow can be directly linked to the extra- and intracellular conductivity and to the tissue capacitance, which is determined by the spacing of the cell membranes. To validate the model, we conducted impedance measurements on healthy and tumorous bladder tissue during ongoing relaxation. After initial deformation, the tissue relaxes and the impedance increases. The proposed model accurately represents these effects and supports the link between fluid flow under mechanical deformation and its impact on tissue impedance. Additionally, we propose a method to compensate for the undesired impedance changes caused by fluid outflow during an intraoperative measurement process. Impedance has the potential to differentiate between healthy and tumorous tissue even under varying mechanical deformation, but this distinction is improved further by the compensation approach. Therefore, electrical impedance measurements show great promise in supporting urologists during endoscopic surgeries.