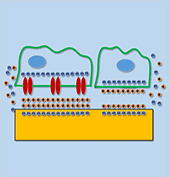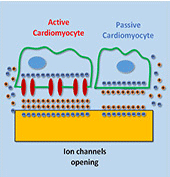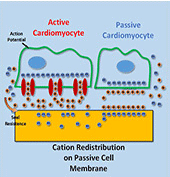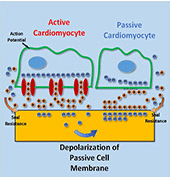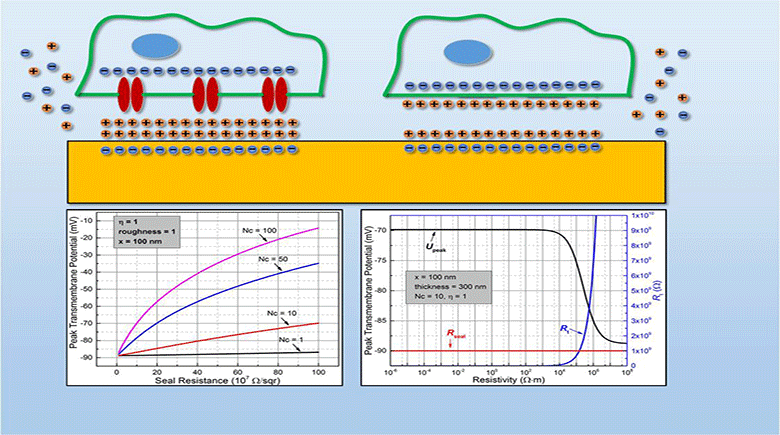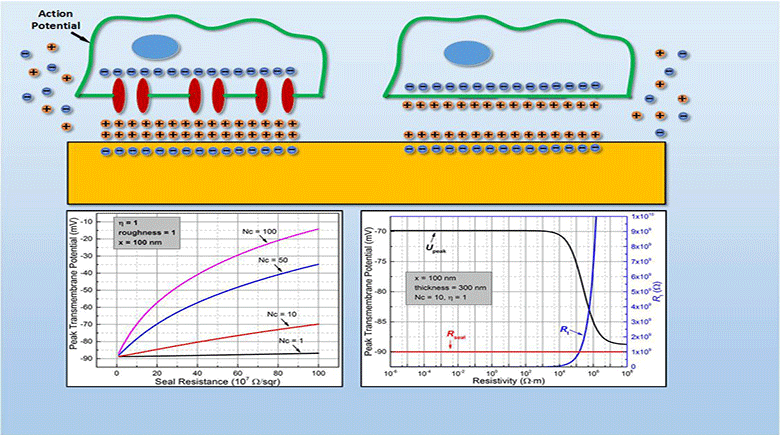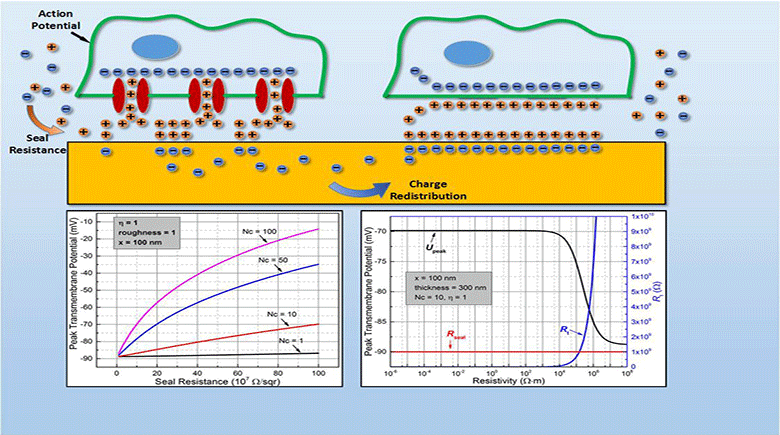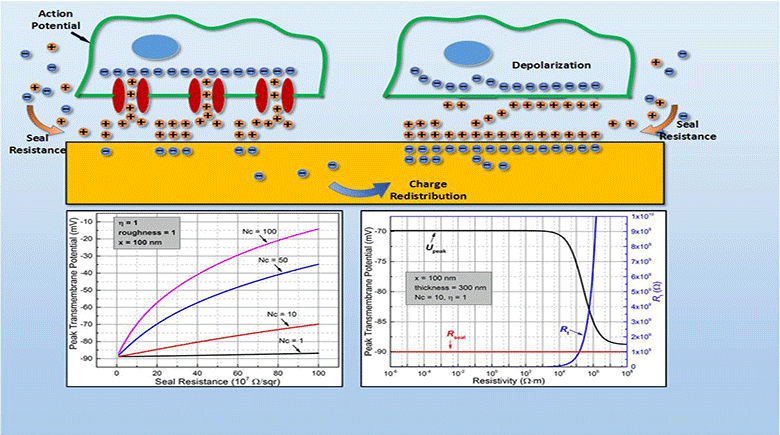
Myocardial infarction, due to interruption of blood supply to part of the myocardium, often results in massive loss of cardiomyocytes that are difficult to regenerate intrinsically, which further impairs synchronization of cardiac tissue and causes cardiac arrhythmias. In parallel with conventional surgical treatment towards heart transplantation, cardiac tissue engineering aims at repairing myocardium function by cellular transplantation and biomaterial scaffolding. Since action potentials of cardiomyocytes are synchronized through electrical coupling, it is generally hypothesized that electrically conductive scaffolds would improve cardiomyocyte function by increasing expression of cardiac genes and gap junctions due to increased cardiac synchronization. Under this principle, various conductive elements including conducting polymers, gold nanowires, or carbon nanotubes have been used to produce electroactive scaffolds, which have demonstrated improvements in cardiac tissue structure, function, and integration into the host myocardium.
In this study, we develop an equivalent-circuit model to theoretically test this hypothesis of conductive material-aided cardiac synchronization and elucidate the underlying biophysical mechanism. In our model systems, the specific problem studied is in the absence of external electrical stimulation how the presence of a conductive element can enhance the electrical synchronization between two cardiomyocyte groups with one group initiating an action potential. By sweeping critical model parameters, we find that cardiac synchronization is most sensitive to the seal resistance, while surface roughness and conductivity of the material have less impact. This work provides a theoretical basis for the rational design of electroactive scaffolds for enhanced cardiac tissue engineering, emphasizing the importance of improving the cell-scaffold adhesion.