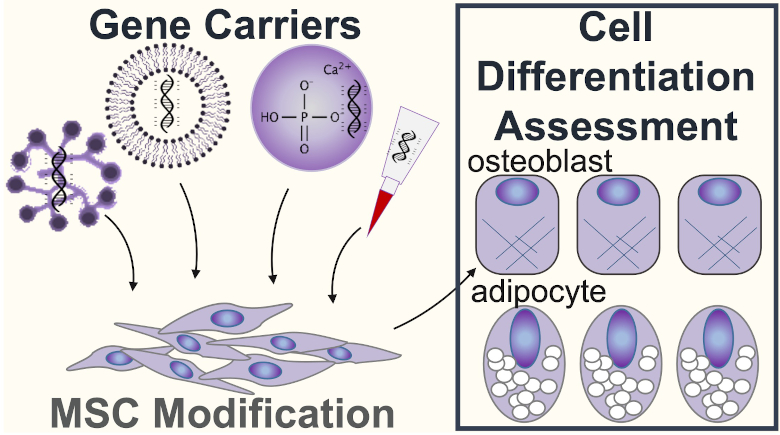
Mesenchymal stem cells (MSCs) have been investigated intensely and are a promising cell source for cell/gene-based therapy, regenerative medicine, and tissue engineering. However, MSCs progressively lose their biological functions after isolation from original tissues and infusion into patients. Introducing a gene to MSCs is a well-known strategy to purposely manipulate the cell fate and further enhance therapeutic performance in cell-based therapy. The current study aimed to investigate the effects of three chemical delivery carriers and one physical delivery approach on MSCs in multipotency and therapeutic gene function. To do this, we present a high productive robotic microinjection system with the ability to process over 3000 cells per hour. We enhanced the efficiency of automatic cell detection and segmentation by using two microinjection modules and deep learning technology.
We optimized the transfection condition of each method. We achieved the highest transfection efficiency of ~60% by microinjection, while lower efficiency of 10-25% by the three others. Robotic microinjection preserved fibroblast-like cell morphology, stress fiber intactness, and mature focal adhesion complex, while PEI caused severe cytotoxicity. No marked differentiation bias was observed after microinjection and cLipo treatment. By contrast, CaP-treated MSCs exhibited excessive osteogenesis, while PEI-treated MSCs showed excessive adipogenesis. We used the robotic microinjection system and the three others to deliver the CRISPR/Cas9-encoding plasmid to knock out the PPARγ gene in MSCs. We demonstrated that robotic microinjection did not interfere with PPARγ function in differentiation commitment. In contrast, the bias in osteo-adipogenic differentiation is exhibited in CaP and PEI-treated MSCs.
Our results indicate that gene delivery vehicles variously disturb MSCs differentiation and interfere with exogenous gene function. Our findings further suggest that robotic microinjection offers a promise of genetically modifying MSCs with minimal disruption on stem cell multipotency and therapeutic gene function.